
Draw the structure of naphthalene and methyl benzene. Draw the structure of 5-(-1-methylpropyl)-4-isopropyldecane. What are neo alkanes?
Answer
577.5k+ views
Hint: Naphthalene is aromatic compound consisting of two benzene rings and has chemical formula as ${{C}_{10}}{{H}_{8}}$, methyl benzene also an aromatic compound and consists of benzene ring along with the alkyl group and has the chemical formula as${{C}_{6}}{{H}_{5}}C{{H}_{3}}$and the compound 5-(-1-methylpropyl)-4-isopropyldecane is an alkane consisting of ten carbon atoms and has isopropyl group at carbon 4 and propyl group at carbon 5 and the neo alkanes consists of four alkyl groups attached to the carbon atom. Now you can easily draw their structures.
Complete step by step solution:
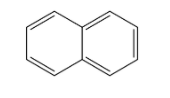
Naphthalene :- It is an aromatic polycyclic hydrocarbon compound which consists of two benzene rings and have the chemical formula as ${{C}_{10}}{{H}_{8}}$ and has the molecular mass as 128. It is produced by the distillation of petrol and is white in colour and volatile in nature and has a very strong odour. The structure of naphthalene is as;
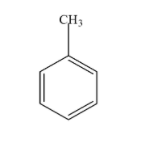
Methyl benzene:- From the name, it is clear that it is an aromatic compound and consists of cyclic ring i.e. the benzene ring and an alkyl group i.e. the methyl group. The carbon atom of the benzene replaces the hydrogen atom with the methyl group and results in the formation of methyl benzene. Its chemical formula is as ${{C}_{6}}{{H}_{5}}C{{H}_{3}}$ and IUPAC name is Toluene. The structure of methyl benzene is as follows;
5-(-1-methylpropyl)-4-isopropyldecane:- We will draw its structure step by step as;
Step I:- First we can see that the compound name ends with decane which means that it is a ten-carbon compound and belongs to the alkane category( dec=10 and ane = alkane)as;
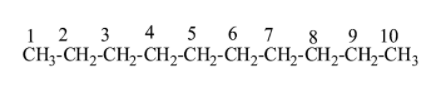
Step II:- Now , we can see that it is given as 4-isopropyl means to the carbon number 4 an isopropyl group is attached to it. Isopropyl group is that group in which the carbon atom consists of two alkyl groups i.e.${{(C{{H}_{3}})}_{2}}CH$. Now the structure (1) becomes as;
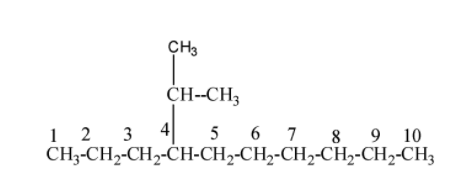
Step III:- Now in the starting of the compound , it is given as 5-(-1-methylpropyl) , it means to the carbon number 5 propyl group i.e. $C{{H}_{3}}-C{{H}_{2}}-C{{H}_{3}}$ is attached and in that propyl group, to carbon number, methyl group i.e. $-C{{H}_{3}}$ is present. Now the structure (2) becomes as;
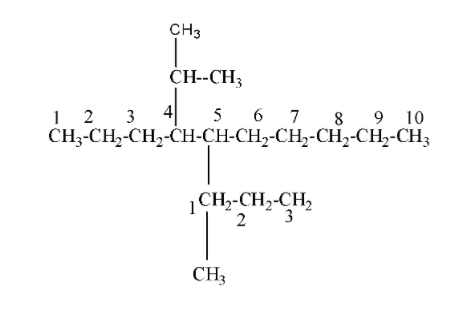
So, this is the overall structure of 5-(-1-methylpropyl)-4-isopropyldecane.
Neo Alkanes:- In this neo means that there are two alkyl groups on the secondary carbon atom i.e. the carbon atom which consists of two alkyl groups and ane means that it is alkane. So, neo alkanes simply means those alkanes in which the carbon atom consists of four alkyl groups. Example : neopentane.
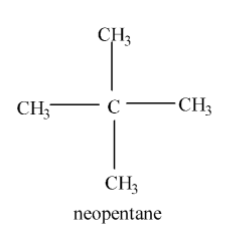
Note: Always keep in mind that if there is an iso word then that means there is one alkyl group attached to the secondary carbon atom and if there is neo word then that means there are two alkyl groups attached to the secondary carbon atom.
Complete step by step solution:
Naphthalene :- It is an aromatic polycyclic hydrocarbon compound which consists of two benzene rings and have the chemical formula as ${{C}_{10}}{{H}_{8}}$ and has the molecular mass as 128. It is produced by the distillation of petrol and is white in colour and volatile in nature and has a very strong odour. The structure of naphthalene is as;

Methyl benzene:- From the name, it is clear that it is an aromatic compound and consists of cyclic ring i.e. the benzene ring and an alkyl group i.e. the methyl group. The carbon atom of the benzene replaces the hydrogen atom with the methyl group and results in the formation of methyl benzene. Its chemical formula is as ${{C}_{6}}{{H}_{5}}C{{H}_{3}}$ and IUPAC name is Toluene. The structure of methyl benzene is as follows;

5-(-1-methylpropyl)-4-isopropyldecane:- We will draw its structure step by step as;
Step I:- First we can see that the compound name ends with decane which means that it is a ten-carbon compound and belongs to the alkane category( dec=10 and ane = alkane)as;
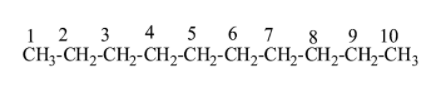
Step II:- Now , we can see that it is given as 4-isopropyl means to the carbon number 4 an isopropyl group is attached to it. Isopropyl group is that group in which the carbon atom consists of two alkyl groups i.e.${{(C{{H}_{3}})}_{2}}CH$. Now the structure (1) becomes as;

Step III:- Now in the starting of the compound , it is given as 5-(-1-methylpropyl) , it means to the carbon number 5 propyl group i.e. $C{{H}_{3}}-C{{H}_{2}}-C{{H}_{3}}$ is attached and in that propyl group, to carbon number, methyl group i.e. $-C{{H}_{3}}$ is present. Now the structure (2) becomes as;

So, this is the overall structure of 5-(-1-methylpropyl)-4-isopropyldecane.
Neo Alkanes:- In this neo means that there are two alkyl groups on the secondary carbon atom i.e. the carbon atom which consists of two alkyl groups and ane means that it is alkane. So, neo alkanes simply means those alkanes in which the carbon atom consists of four alkyl groups. Example : neopentane.

Note: Always keep in mind that if there is an iso word then that means there is one alkyl group attached to the secondary carbon atom and if there is neo word then that means there are two alkyl groups attached to the secondary carbon atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




