
What is the electron configuration for a neutral atom of manganese?
Answer
529.2k+ views
Hint: Since electronic configuration shows how electrons are arranged in an atom or a molecule, we need to understand how the filling of electrons in an atom is governed and what are quantum numbers. The atomic number of Manganese (Mn) is 25.
Complete answer:
An atom which does not contain any charge and there is no loss of electrons or gain of electrons is called a neutral atom.
In a neutral atom, the number of electrons is equal to the number of protons in the atom i.e., the atomic number of the element. Hence the number of electrons in neutral manganese atoms is 25.
Now the electrons in an atom in the ground state are arranged in different shells and subshells the following sequence according to the diagonal rule.
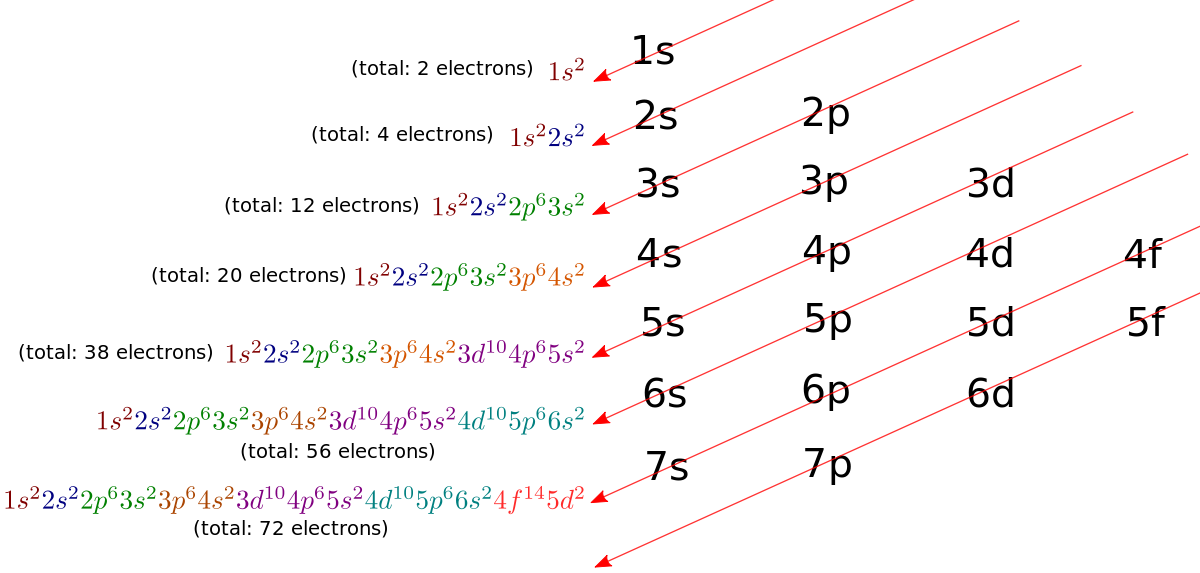
Here, the integer numbers 1, 2, 3... represent the shells, in increasing order of their energy, and are known as the principal quantum number.
n=1,2,3,4....
The ${{n}^{th}}$ shell of an atom can have a maximum $2{{n}^{2}}$ number of electrons.
So, maximum number of electrons in shell
(K) n = 1 is 2
(L) n = 2 is 8
(M) n = 3 is 18
(N) n = 4 is 32
s, p, d, f… represent the subshells in a shell and are given by azimuthal quantum number.
$\ell $=0 to (n-1)
In a subshell the maximum number of electrons is given by 2(2$\ell $+1).
So, the maximum number of electrons in the subshell
($\ell $= 0) s is 2
($\ell $= 1) p is 6
($\ell $= 2) d is 10
($\ell $= 3) f is 14
So, its electronic configuration in ground state will be
\[\begin{align}
& ^{25}Mn=1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{5}} \\
& or{{\text{ }}^{25}}Mn=[Ar]3{{d}^{5}}4{{s}^{2}} \\
\end{align}\]
Additional information:
The magnetic quantum number (m) gives the number of orbitals in a subshell.
m= -$\ell $ to +$\ell $
Hence,
s subshell has 1 orbital
p subshell has 3 orbitals
d subshell has 5 orbitals
f subshell has 7 orbitals
Each orbital can have two electrons of the opposite spin (positive spin-up $(+\dfrac{1}{2})$ or a negative spin-down $(-\dfrac{1}{2})$) as stated by the spin quantum number.
Note:
It should be noted that manganese has an exactly half-filled d-subshell. This is due to the fact that the electron that is supposed to be the 6th electron in the d orbitals goes to the s orbital to fill the outermost s shell.
This results in extra stability of the manganese atom in the ground state because of
-Symmetric configuration and distribution of electrons.
- The maximum possible number of exchanges of electrons between orbitals and hence maximum exchange energy.
Complete answer:
An atom which does not contain any charge and there is no loss of electrons or gain of electrons is called a neutral atom.
In a neutral atom, the number of electrons is equal to the number of protons in the atom i.e., the atomic number of the element. Hence the number of electrons in neutral manganese atoms is 25.
Now the electrons in an atom in the ground state are arranged in different shells and subshells the following sequence according to the diagonal rule.

Here, the integer numbers 1, 2, 3... represent the shells, in increasing order of their energy, and are known as the principal quantum number.
n=1,2,3,4....
The ${{n}^{th}}$ shell of an atom can have a maximum $2{{n}^{2}}$ number of electrons.
So, maximum number of electrons in shell
(K) n = 1 is 2
(L) n = 2 is 8
(M) n = 3 is 18
(N) n = 4 is 32
s, p, d, f… represent the subshells in a shell and are given by azimuthal quantum number.
$\ell $=0 to (n-1)
In a subshell the maximum number of electrons is given by 2(2$\ell $+1).
So, the maximum number of electrons in the subshell
($\ell $= 0) s is 2
($\ell $= 1) p is 6
($\ell $= 2) d is 10
($\ell $= 3) f is 14
So, its electronic configuration in ground state will be
\[\begin{align}
& ^{25}Mn=1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{5}} \\
& or{{\text{ }}^{25}}Mn=[Ar]3{{d}^{5}}4{{s}^{2}} \\
\end{align}\]
Additional information:
The magnetic quantum number (m) gives the number of orbitals in a subshell.
m= -$\ell $ to +$\ell $
Hence,
s subshell has 1 orbital
p subshell has 3 orbitals
d subshell has 5 orbitals
f subshell has 7 orbitals
Each orbital can have two electrons of the opposite spin (positive spin-up $(+\dfrac{1}{2})$ or a negative spin-down $(-\dfrac{1}{2})$) as stated by the spin quantum number.
Note:
It should be noted that manganese has an exactly half-filled d-subshell. This is due to the fact that the electron that is supposed to be the 6th electron in the d orbitals goes to the s orbital to fill the outermost s shell.
This results in extra stability of the manganese atom in the ground state because of
-Symmetric configuration and distribution of electrons.
- The maximum possible number of exchanges of electrons between orbitals and hence maximum exchange energy.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




