
What should the electron dot diagram for iodine look like?
Answer
528.6k+ views
Hint: The electron dot diagram is the diagram which clearly shows the bonding between the atoms of a molecule along with the lone pairs of electrons, if they exist in the molecule. Iodine is the molecule consisting of two atoms of iodine bonded together satisfying both the planes.
Complete answer:
Let us study the concept;
The electron dot diagram depends purely upon the number of valence electrons present in an atom. The valence electrons or the electrons present in the outermost shell is being represented in the electron dot formula. For this, we need to know the electronic configuration of the atom comprising the molecule.
Here, we have 2 atoms of iodine making up an iodine atom. Thus, the electronic configuration of iodine with atomic number of 53 is given as;
$I\to \left[ Kr \right]4{{d}^{10}}5{{s}^{2}}5{{p}^{5}}$
Thus, we can see that it has 7 valence electrons present which will be present in the electron dot diagram. It needs an electron to complete its octet. Thus, both atoms will share the electrons and will form a stable molecule i.e. ${{I}_{2}}$ . The electron dot structure is represented as;
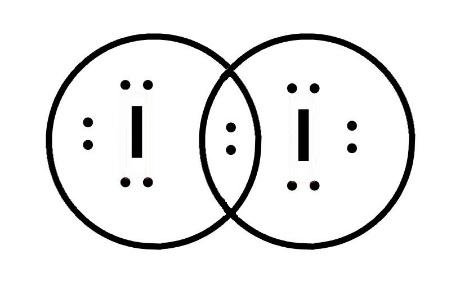
Note:
Do note that the electron dot diagram or Lewis structure or Lewis dot diagram or Lewis dot structure or Lewis dot formula or electron dot structure or Lewis electron dot structure is the same. The same concept is represented by different names, so do not get confused.
Complete answer:
Let us study the concept;
The electron dot diagram depends purely upon the number of valence electrons present in an atom. The valence electrons or the electrons present in the outermost shell is being represented in the electron dot formula. For this, we need to know the electronic configuration of the atom comprising the molecule.
Here, we have 2 atoms of iodine making up an iodine atom. Thus, the electronic configuration of iodine with atomic number of 53 is given as;
$I\to \left[ Kr \right]4{{d}^{10}}5{{s}^{2}}5{{p}^{5}}$
Thus, we can see that it has 7 valence electrons present which will be present in the electron dot diagram. It needs an electron to complete its octet. Thus, both atoms will share the electrons and will form a stable molecule i.e. ${{I}_{2}}$ . The electron dot structure is represented as;

Note:
Do note that the electron dot diagram or Lewis structure or Lewis dot diagram or Lewis dot structure or Lewis dot formula or electron dot structure or Lewis electron dot structure is the same. The same concept is represented by different names, so do not get confused.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




