
How many electrons are in the valence shells of
a) $Be$ In \[BeC{{l}_{2}}\]
b) $B$ In \[BC{{l}_{3}}\]
c) $H$ In \[{{H}_{2}}O\]
Answer
558.9k+ views
Hint:We know that a valence electron is an electron that is associated with an atom, and apparently that can participate in the formation of a chemical bond so we can form it in a single covalent bond, both atoms in the bond contribute one valence electron in order to form a shared pair. The key attribute is given by presence of valence electrons can determine the element's chemical properties and whether it may bond with other elements: For a main group element, we have to keep this in consideration that a valence electron can only be in the outermost electron shell.
Complete answer:
We know that Beryllium Be has $4$ valence electrons in the compound \[BeC{{l}_{2}}\].
$Be$ has $2$ valence electrons of its own and shares $1$ electrons with each of the two chlorine atoms. Electrons in Valence shells of $Be$ in \[BeC{{l}_{2}}\] is given by:

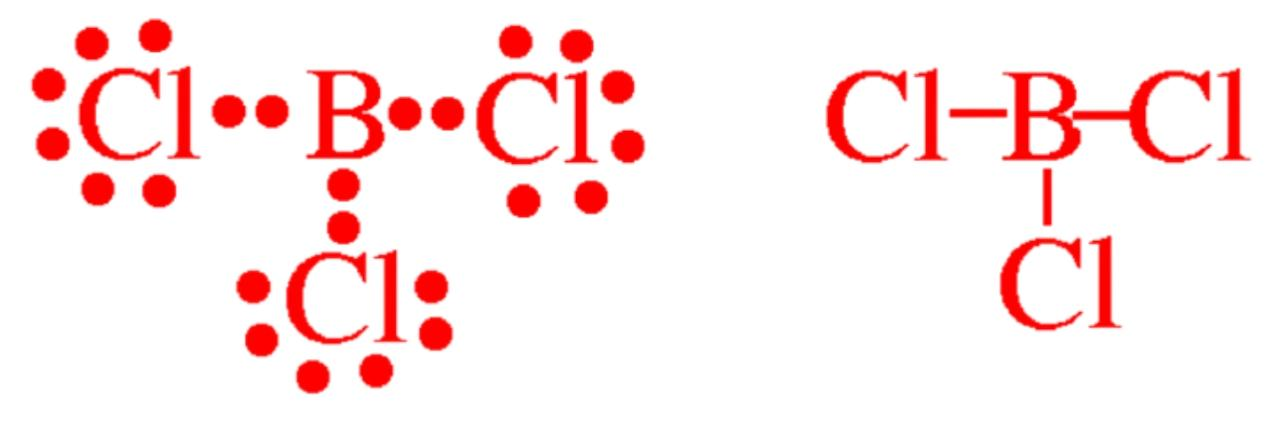
b) Similarly Boron $B$ has $6$ valence electrons in the compound Boron Trichloride $BC{{l}_{3}}$
$B$ has $3$ valence electrons of its own and shares $1$ electrons with each of the three chlorine atoms. An electron in Valence shells of $B$ in $BC{{l}_{3}}$ is given by:
c) Likewise we have $H$ in ${{H}_{2}}O$ where each $H$ atom in ${{H}_{2}}O$
${{H}_{2}}O$ has 2 valence electrons. Each $H$ atom has $1$ valence electron of its own and shares $1$ electron with the oxygen atom. An electron in Valence shells of $H$ in ${{H}_{2}}O$ is given by:

Note:Also note that thiols are compounds that are structurally identical to alcohols except that they replace the oxygen atom in the hydroxyl group with a sulfur atom. Likewise, sulfides are structurally identical to ethers, but they replace the oxygen atom with a sulfur atom, as shown below.
Complete answer:
We know that Beryllium Be has $4$ valence electrons in the compound \[BeC{{l}_{2}}\].
$Be$ has $2$ valence electrons of its own and shares $1$ electrons with each of the two chlorine atoms. Electrons in Valence shells of $Be$ in \[BeC{{l}_{2}}\] is given by:

b) Similarly Boron $B$ has $6$ valence electrons in the compound Boron Trichloride $BC{{l}_{3}}$
$B$ has $3$ valence electrons of its own and shares $1$ electrons with each of the three chlorine atoms. An electron in Valence shells of $B$ in $BC{{l}_{3}}$ is given by:

c) Likewise we have $H$ in ${{H}_{2}}O$ where each $H$ atom in ${{H}_{2}}O$
${{H}_{2}}O$ has 2 valence electrons. Each $H$ atom has $1$ valence electron of its own and shares $1$ electron with the oxygen atom. An electron in Valence shells of $H$ in ${{H}_{2}}O$ is given by:

Note:Also note that thiols are compounds that are structurally identical to alcohols except that they replace the oxygen atom in the hydroxyl group with a sulfur atom. Likewise, sulfides are structurally identical to ethers, but they replace the oxygen atom with a sulfur atom, as shown below.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




