
How many electrons are used in bonding the Lewis structure of $ {C_2}O_4^{2 - } $ (oxalate) ions?
Answer
479.1k+ views
Hint: Each covalent and ionic bond is formed by the contribution of two electrons. So the total number of bonds has to be figured out using the correct lewis structure. Then the number of electrons involved in bonding can be calculated by multiplying the number of bonds by two.
Complete answer:
First, we need to figure out the lewis structure of the oxalate ion, which is –
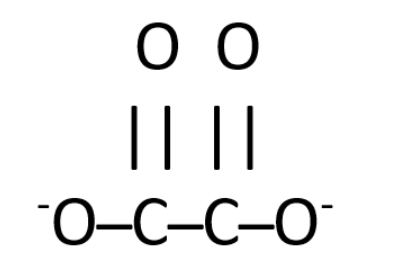
As the structure of oxalate ions suggests, there are a total of seven bonds present in one ion. As there are seven bonds so the number of electrons bonding will be twice of it, i.e. fourteen.
Hence, the correct answer is fourteen electrons are used in bonding the Lewis structure of $ {C_2}O_4^{2 - } $ (oxalate) ions.
Note:
Oxalate ion is the conjugate base of oxalic acid, it reacts with metals and hydrogen to form compounds like sodium oxalate and oxalic acid. Calcium oxalate is the chief component of kidney stones. These are the product of incomplete oxidation of carbohydrates.
Complete answer:
First, we need to figure out the lewis structure of the oxalate ion, which is –
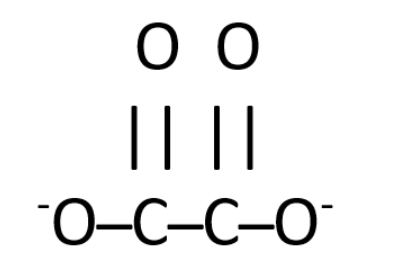
As the structure of oxalate ions suggests, there are a total of seven bonds present in one ion. As there are seven bonds so the number of electrons bonding will be twice of it, i.e. fourteen.
Hence, the correct answer is fourteen electrons are used in bonding the Lewis structure of $ {C_2}O_4^{2 - } $ (oxalate) ions.
Note:
Oxalate ion is the conjugate base of oxalic acid, it reacts with metals and hydrogen to form compounds like sodium oxalate and oxalic acid. Calcium oxalate is the chief component of kidney stones. These are the product of incomplete oxidation of carbohydrates.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




