
Electrons revolve around the ______________in different energy levels or shells and each shell is associated with definite energy.
A) nucleus
B) Orbits
C) Orbitals
D) None of the above
Answer
578.1k+ views
Hint: The atom electron model is considered the same as that of the planetary model. In the planetary model, the sun is at the centre and planets revolve around in a fixed path. Likewise, in atoms positive charge mass is situated at the centre of the atom and negatively charged electrons revolve around it.
Complete step by step answer:
-In 1911, Ernest Rutherford performed an alpha –ray scattering experiment. According to the experiment the atomic mass is located as the tiny point which is extremely dense. This mass is known as the nucleus. This is a positive charge value.
-The nucleus is composed of two particles: proton and neutron. The proton is positively charged while neutrons are neutral. Two protons may result in forces of repulsion. Neutrons balance out or remove the force of attraction and thus stabilize the nucleus. Since neutrons are charged less species, the nucleus is measured as a positive charge.
-We know that an atom follows electrical neutrality thus there must be a negative charge.
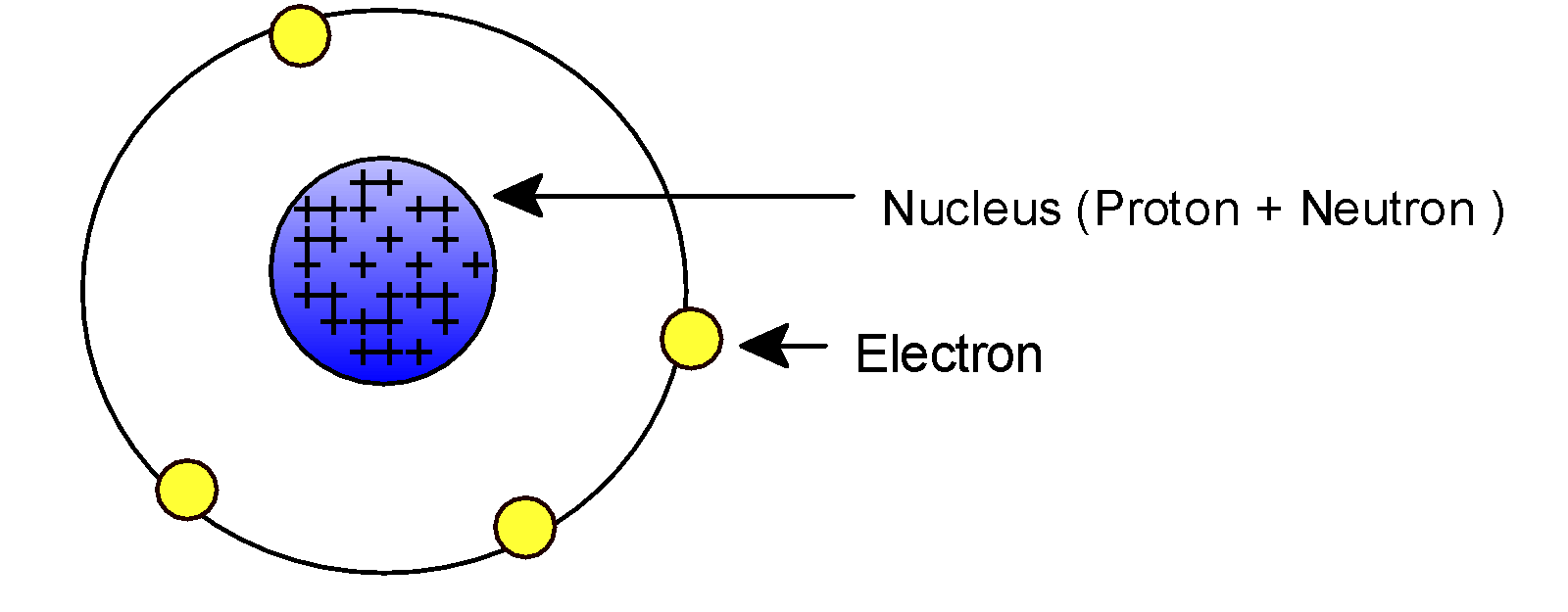
-There is an electrostatic force of attraction between negative charge (electron) and positive charge (nucleus) which in turn offers a necessary centripetal force so that electrons revolve around the nucleus in a definite path.
Therefore, electrons revolve around the nucleus in different energy levels or shells, and each shell is associated with definite energy.
Note: According to the postulate of Bohr's model of atom the electrons in the atom revolve around the nucleus only in certain selected circular paths. As long as the electrons remain in the circular path it neither absorbs energy nor loses it. This circular path having definite energy i.e. with a definite whole number of quanta is known as the orbit.
Complete step by step answer:
-In 1911, Ernest Rutherford performed an alpha –ray scattering experiment. According to the experiment the atomic mass is located as the tiny point which is extremely dense. This mass is known as the nucleus. This is a positive charge value.
-The nucleus is composed of two particles: proton and neutron. The proton is positively charged while neutrons are neutral. Two protons may result in forces of repulsion. Neutrons balance out or remove the force of attraction and thus stabilize the nucleus. Since neutrons are charged less species, the nucleus is measured as a positive charge.
-We know that an atom follows electrical neutrality thus there must be a negative charge.

-There is an electrostatic force of attraction between negative charge (electron) and positive charge (nucleus) which in turn offers a necessary centripetal force so that electrons revolve around the nucleus in a definite path.
Therefore, electrons revolve around the nucleus in different energy levels or shells, and each shell is associated with definite energy.
Note: According to the postulate of Bohr's model of atom the electrons in the atom revolve around the nucleus only in certain selected circular paths. As long as the electrons remain in the circular path it neither absorbs energy nor loses it. This circular path having definite energy i.e. with a definite whole number of quanta is known as the orbit.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




