
Elements in the same group show the same valency. Give scientific reason.
Answer
570.6k+ views
Hint: Valency of an electron is the number of electrons which needed to be lost or gained in order for the octet to be complete. Valency is a function of valence electrons. Valence electrons are the same for the elements belonging to the same group.
Complete step by step answer:
Let us look at the periodic table here,
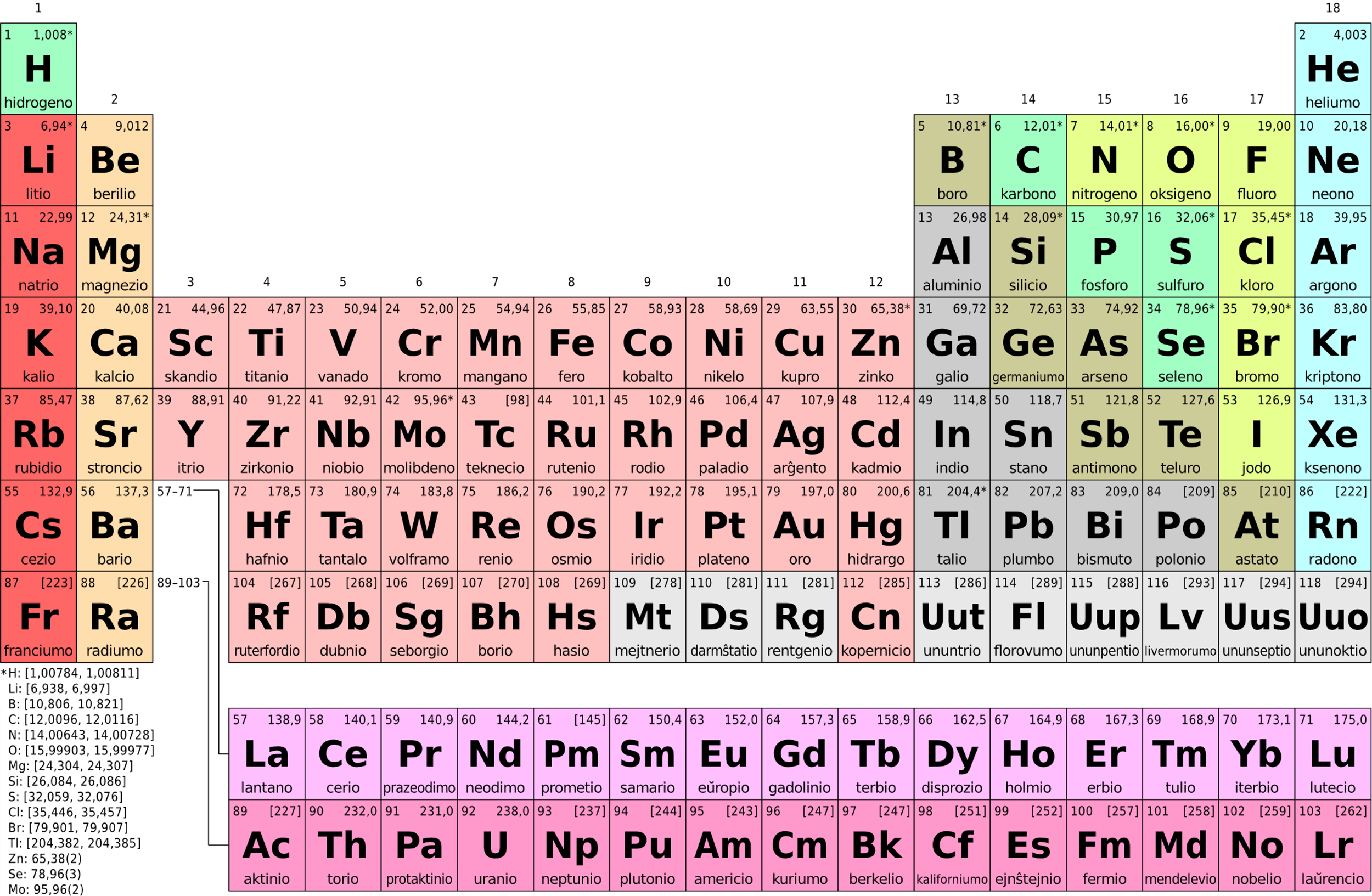 Notice, the leftmost group, it starts with Hydrogen.
Notice, the leftmost group, it starts with Hydrogen.
This group has elements which are called alkali metals.
Now, this is group $1$
So all the elements in this group will have one electron in the outermost shell
Let us take a few examples
Sodium $Na = 1{s^2}2{s^2}2{p^6}3{s^1}$
Potassium $K = 1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^1}$
Note, that the number of electrons in the last shell are the same.
We know the maximum possible electrons in the s shell is two. So these shells are half filled.
In order to attain octet configuration, which will be eight electrons in the last shell, it is easier to lose one electron and attain octet in the previous shell rather than gaining additional seven electrons and attaining octet in the last shell.
So, the excited state configuration is:
$N{a^ + } = 1{s^2}2{s^2}2{p^6}3{s^0}$
${K^ + } = 1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^0}$
So their valency is one.
This principle is true for every group. Since, every group has elements with the same outer shell configuration. They have the same valency.
Note: A similarity in the valency is also responsible for the chemical properties of the elements. The oxides of the first group elements are basic in nature. They may differ in the magnitude of basicity however.
Elements in the same group have similar types of chemical reactions , which makes it easier to predict the nature of an element if its electronic configuration is known.
Complete step by step answer:
Let us look at the periodic table here,

This group has elements which are called alkali metals.
Now, this is group $1$
So all the elements in this group will have one electron in the outermost shell
Let us take a few examples
Sodium $Na = 1{s^2}2{s^2}2{p^6}3{s^1}$
Potassium $K = 1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^1}$
Note, that the number of electrons in the last shell are the same.
We know the maximum possible electrons in the s shell is two. So these shells are half filled.
In order to attain octet configuration, which will be eight electrons in the last shell, it is easier to lose one electron and attain octet in the previous shell rather than gaining additional seven electrons and attaining octet in the last shell.
So, the excited state configuration is:
$N{a^ + } = 1{s^2}2{s^2}2{p^6}3{s^0}$
${K^ + } = 1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^0}$
So their valency is one.
This principle is true for every group. Since, every group has elements with the same outer shell configuration. They have the same valency.
Note: A similarity in the valency is also responsible for the chemical properties of the elements. The oxides of the first group elements are basic in nature. They may differ in the magnitude of basicity however.
Elements in the same group have similar types of chemical reactions , which makes it easier to predict the nature of an element if its electronic configuration is known.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




