
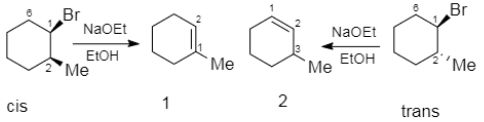
Elimination reaction of cis- and trans $-1-$ Bromo $-2-$ methylcyclohexanes with the \[NaOEt\] in \[EtOH\] can give the same or different main product, \[1-\] methylcyclohexene (1) or \[3-\]methylcyclohexene (2). Which of (a)-(d) indicates the main products?
A.$1$ from the cis and $2$ from the trans substrate
B.$2$ from the cis and $1$ from the trans substrate
C.$2$ both from the cis and the trans substrate
D.$1$ both from the cis and the trans substrate

Answer
547.5k+ views
Hint:The chair conformation of the cyclohexane explains the axial and equatorial bonds which are present in the cis- and trans- structures of a substituted cyclohexane.
These confirmation can help us predict the product of an elimination reaction and the position of the double bonds in the products.
Complete step-by-step answer:The two reactants cis and the trans $-1-$ Bromo $-2-$ methylcyclohexanes after individually reacting with sodium ethanoate and ethanol. Now, as we can see there are two reactions which are indicated in the question, in which the reactants differ only in their special arrangements. One of them hence becomes cis and the other becomes trans. In this reaction as we can see that the bromine is the leaving group and the removal of the bromine group would establish the formation of a double bond between the two adjacent carbons.
Unlike structures having an open chain, the cyclic compounds are usually known to have restricted the spatial arrangements of substituents of the ring to relatively lesser orientations. As a consequence of this, reactions which are conducted on these kind of substrates often give us the information about the orientation which is preferred by reactant species in their transition state.
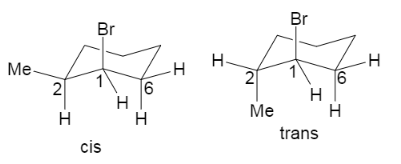
Now, as we can see in the chair conformations the substituents which are present at the positions one and two, takes equatorial and axial position in the cis form, but both of the substituents are in equatorial positions or both are in axial positions in the case of trans form. Hence in the case of cis form, the bromine has the presence of two axial hydrogen which is present in its adjacent carbons $C2$ and $C6$. But in case of the trans structure, there is only one axial hydrogen adjacent to the axial bromine, which is $C6$.
So, the correct option would be A, which says $1$ from the cis and $2$ from the trans substrate.
Note:When a trans cyclohexane undergoes elimination reaction it develops a double bond in the $2-3$ position of the other substituent.
But when a cis cyclohexane undergoes elimination reaction, it develops a double bond in the adjacent position, and this can be understood by the use of chair conformation of the cyclohexane.
These confirmation can help us predict the product of an elimination reaction and the position of the double bonds in the products.
Complete step-by-step answer:The two reactants cis and the trans $-1-$ Bromo $-2-$ methylcyclohexanes after individually reacting with sodium ethanoate and ethanol. Now, as we can see there are two reactions which are indicated in the question, in which the reactants differ only in their special arrangements. One of them hence becomes cis and the other becomes trans. In this reaction as we can see that the bromine is the leaving group and the removal of the bromine group would establish the formation of a double bond between the two adjacent carbons.
Unlike structures having an open chain, the cyclic compounds are usually known to have restricted the spatial arrangements of substituents of the ring to relatively lesser orientations. As a consequence of this, reactions which are conducted on these kind of substrates often give us the information about the orientation which is preferred by reactant species in their transition state.

Now, as we can see in the chair conformations the substituents which are present at the positions one and two, takes equatorial and axial position in the cis form, but both of the substituents are in equatorial positions or both are in axial positions in the case of trans form. Hence in the case of cis form, the bromine has the presence of two axial hydrogen which is present in its adjacent carbons $C2$ and $C6$. But in case of the trans structure, there is only one axial hydrogen adjacent to the axial bromine, which is $C6$.
So, the correct option would be A, which says $1$ from the cis and $2$ from the trans substrate.
Note:When a trans cyclohexane undergoes elimination reaction it develops a double bond in the $2-3$ position of the other substituent.
But when a cis cyclohexane undergoes elimination reaction, it develops a double bond in the adjacent position, and this can be understood by the use of chair conformation of the cyclohexane.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




