
How is ethanol prepared by methanal by using Grignard reagent?
Answer
583.5k+ views
Hint: As we know that the functional group in ethanol is alcohol and in methanal the functional group is aldehyde (carbonyls). The carbonyls contain carbon and oxygen, so the carbon in carbonyls are very reactive.
Complete step by step answer:
The methanal is having aldehyde as a functional group and represented as \[HCHO\] . The carbonyl carbon is very electrophilic in nature in aldehydes and can be attacked by any nucleophile.
The Grignard reagent is used to convert aldehydes into alcohol. It is represented as \[CH \equiv {C^ - }N{a^ + }\]where \[R\]is the alkyl group and \[X\]represents the halogen element.
The mechanism involved for the conversion of methanal to ethanol is nucleophilic addition reaction as shown below.
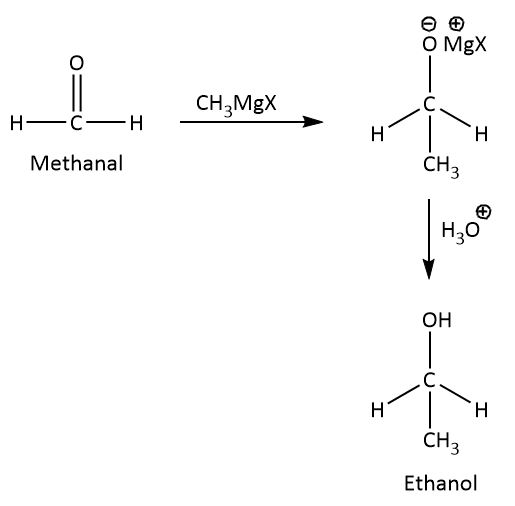
When methanal reacts with methyl magnesium halide, the carbonyl carbon adds methyl group because the magnesium is an alkaline earth metal it gives its electron to methyl group and hence methyl acts as a nucleophile and electropositive magnesium attracts towards carbonyl oxygen.
By the hydrolysis of the intermediate we will get ethanol as a product.
Note:
The Grignard reagent is also known as organometallic compound and has several uses such as it can also act as proton abstractor for ketones which have beta hydrogen and it also can be used for the donation of hydride to ketones.
Complete step by step answer:
The methanal is having aldehyde as a functional group and represented as \[HCHO\] . The carbonyl carbon is very electrophilic in nature in aldehydes and can be attacked by any nucleophile.
The Grignard reagent is used to convert aldehydes into alcohol. It is represented as \[CH \equiv {C^ - }N{a^ + }\]where \[R\]is the alkyl group and \[X\]represents the halogen element.
The mechanism involved for the conversion of methanal to ethanol is nucleophilic addition reaction as shown below.

When methanal reacts with methyl magnesium halide, the carbonyl carbon adds methyl group because the magnesium is an alkaline earth metal it gives its electron to methyl group and hence methyl acts as a nucleophile and electropositive magnesium attracts towards carbonyl oxygen.
By the hydrolysis of the intermediate we will get ethanol as a product.
Note:
The Grignard reagent is also known as organometallic compound and has several uses such as it can also act as proton abstractor for ketones which have beta hydrogen and it also can be used for the donation of hydride to ketones.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




