
Ethoxy benzene is called PHENETOLE. How many more ethers can be drawn for the same formula?
Answer
517.2k+ views
Hint: We know that Phenetole is ethyl phenyl ether; that is, an ethyl group and a phenyl group on either sides of an ether group. o-Cresol is also known as methyl phenol, and consists of a methyl group on the ortho position of phenol.
Complete answer:
First let us try to draw the structure of the phenetole. This compound is ether, and thus, consists of an oxygen group sandwiched in between two other groups. In phenetole, the first such group is a phenyl group, and the next group is an ethyl group. Thus, it is also known as ethyl phenyl ether. Now let us look at the second compound given and try to draw its structure. o-cresol is an isomer of cresol, and has the IUPAC name methyl phenol. Thus, it is a phenol derivative and its structure consists of a methyl group at the ortho position of phenol. Phenetole is a colourless oily liquid with a distinctive odour. It is mainly used as a research chemical and as an intermediate in the perfume industry. O-cresol is a colourless solid that is extracted from coal tar. It mainly acts as a starting material in the production of other compounds.
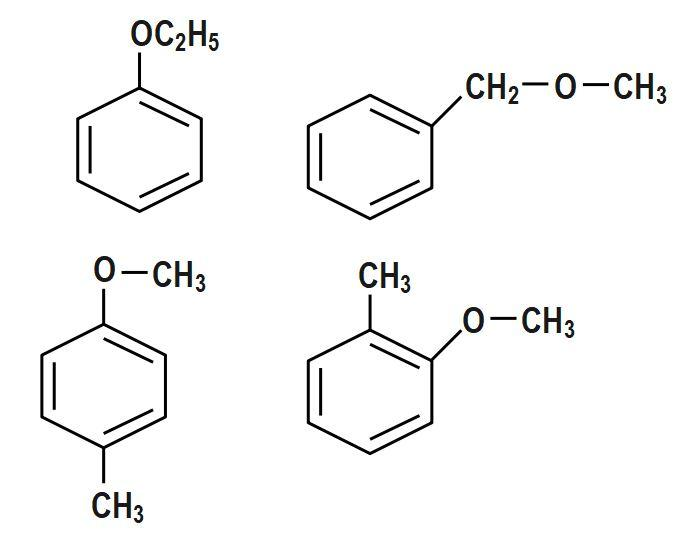
And $ {{H}_{3}}C-HC=C=CH-O-CH=CH-CH=C{{H}_{2}} $ .
Note:
Remember that the structure of compounds is drawn according to the IUPAC name, which specifies the preference of placing functional groups in the compound. Note that the other isomers of cresol are m-cresol and p-cresol. Isomers are compounds with the same molecular formula but different structural formula.
Complete answer:
First let us try to draw the structure of the phenetole. This compound is ether, and thus, consists of an oxygen group sandwiched in between two other groups. In phenetole, the first such group is a phenyl group, and the next group is an ethyl group. Thus, it is also known as ethyl phenyl ether. Now let us look at the second compound given and try to draw its structure. o-cresol is an isomer of cresol, and has the IUPAC name methyl phenol. Thus, it is a phenol derivative and its structure consists of a methyl group at the ortho position of phenol. Phenetole is a colourless oily liquid with a distinctive odour. It is mainly used as a research chemical and as an intermediate in the perfume industry. O-cresol is a colourless solid that is extracted from coal tar. It mainly acts as a starting material in the production of other compounds.

And $ {{H}_{3}}C-HC=C=CH-O-CH=CH-CH=C{{H}_{2}} $ .
Note:
Remember that the structure of compounds is drawn according to the IUPAC name, which specifies the preference of placing functional groups in the compound. Note that the other isomers of cresol are m-cresol and p-cresol. Isomers are compounds with the same molecular formula but different structural formula.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




