
Ethyl benzoate can be prepared from benzoic acid by using
A .Ethyl alcohol
B. Ethyl alcohol and dry $ HCl $
C. Ethyl chloride
D. Sodium ethoxide
Answer
539.7k+ views
Hint :We know that reactions of carboxylic acids involving $ C-OH $ bond cleavage definition, Reactions involving cleavage of $ C-OH $ Bond are Formation of anhydride, Esterification, Reaction with $ ~PC{{l}_{3}},\text{ }PC{{l}_{5}},\text{ }SOC{{l}_{5}} $ Reaction with ammonia, Preparation of esters from carboxylic acids
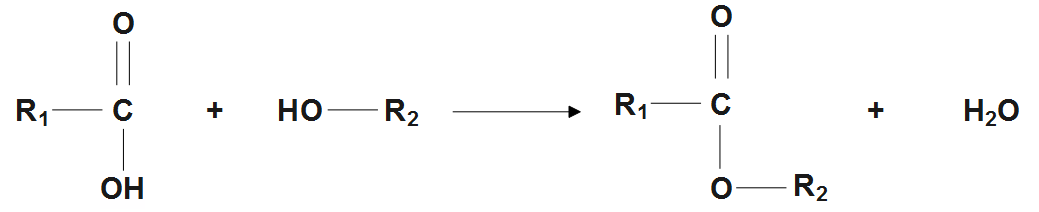
Carboxylic acids react with alcohols in presence of an acid catalyst to give esters and water.
Complete Step By Step Answer:
Ethyl benzoate is prepared by reacting benzoic acid and ethanol in the presence of dry $ HCl. $ This reaction is known as esterification reaction
$ {{C}_{6}}{{H}_{5}}COOH+{{C}_{2}}{{H}_{5}}OH\underset{{}}{\overset{DryHCl}{\longleftrightarrow}}{{C}_{6}}{{H}_{5}}COO{{C}_{2}}{{H}_{5}}+{{H}_{2}}O $
$ Benzoic Acid+Ethanol\overset{DryHCl}{leftrightarrows}Ethyl Benzoate+Water $
This reaction proceeds with equilibrium. Therefore, $ {{H}_{2}}O $ is continuously removed from the reaction. For preparation of ester products. Benzoic acid was first described. The name was derived from gum benzoin. It is a crystalline organic compound. It is white and belongs to the family of carboxylic acid. It was first prepared from coal tar. It is used in various ways, like manufacturing of insect repellents, below are some uses of benzoic acid in our life
Ethyl benzoate is used as a perfume scent. It acts as a food flavoring agent. It is an active component of artificial fruit flavors. Further, it is used in cosmetics and personal care products as fragrance ingredients and preservatives
Therefore, Correct answer is Option B i.e. Ethyl benzoate can be prepared from benzoic acid by using ethyl alcohol and dry $ HCl. $
Note :
Note that the preparation of amines from carboxylic acids, Carboxylic acids can be converted to amines through the Schmidt reaction. The acid-catalyzed reaction of hydrogen azide with carboxylic acids gives corresponding amines with one less carbon atom.
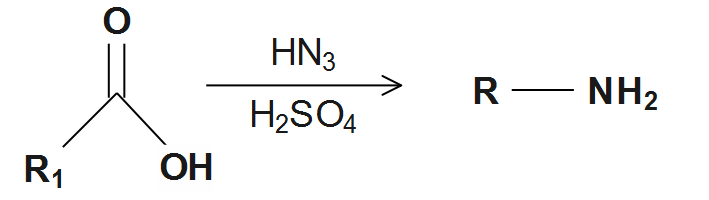

Carboxylic acids react with alcohols in presence of an acid catalyst to give esters and water.
Complete Step By Step Answer:
Ethyl benzoate is prepared by reacting benzoic acid and ethanol in the presence of dry $ HCl. $ This reaction is known as esterification reaction
$ {{C}_{6}}{{H}_{5}}COOH+{{C}_{2}}{{H}_{5}}OH\underset{{}}{\overset{DryHCl}{\longleftrightarrow}}{{C}_{6}}{{H}_{5}}COO{{C}_{2}}{{H}_{5}}+{{H}_{2}}O $
$ Benzoic Acid+Ethanol\overset{DryHCl}{leftrightarrows}Ethyl Benzoate+Water $
This reaction proceeds with equilibrium. Therefore, $ {{H}_{2}}O $ is continuously removed from the reaction. For preparation of ester products. Benzoic acid was first described. The name was derived from gum benzoin. It is a crystalline organic compound. It is white and belongs to the family of carboxylic acid. It was first prepared from coal tar. It is used in various ways, like manufacturing of insect repellents, below are some uses of benzoic acid in our life
Ethyl benzoate is used as a perfume scent. It acts as a food flavoring agent. It is an active component of artificial fruit flavors. Further, it is used in cosmetics and personal care products as fragrance ingredients and preservatives
Therefore, Correct answer is Option B i.e. Ethyl benzoate can be prepared from benzoic acid by using ethyl alcohol and dry $ HCl. $
Note :
Note that the preparation of amines from carboxylic acids, Carboxylic acids can be converted to amines through the Schmidt reaction. The acid-catalyzed reaction of hydrogen azide with carboxylic acids gives corresponding amines with one less carbon atom.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




