
Ethylene on reaction with alkaline $Kmn{O_4}$ gives:
A. Glycerol
B. Acetic acid
C. Glycol
D. Ethyl alcohol
Answer
531.2k+ views
Hint: Potassium permanganate is an oxidizing agent and due to the presence of the pi bond in an alkene, it gets readily oxidized into 1,2-diol or 1,2-glycols with cold diluted or alkaline $Kmn{O_4}$ while the $Kmn{O_4}$ is itself reduced to $Mn{O_2}$.
Complete step by step answer:
Since potassium permanganate is an oxidizing agent it gives oxygen in neutral as well as in an alkaline medium that oxygen obtained is oxidized the alkene into 1,2-diol. When ethylene or ethene reacts with alkaline potassium permanganate it gives ethane-1,2-diol or ethylene glycol.
The reaction of ethylene with alkaline $Kmn{O_4}$ is given as:
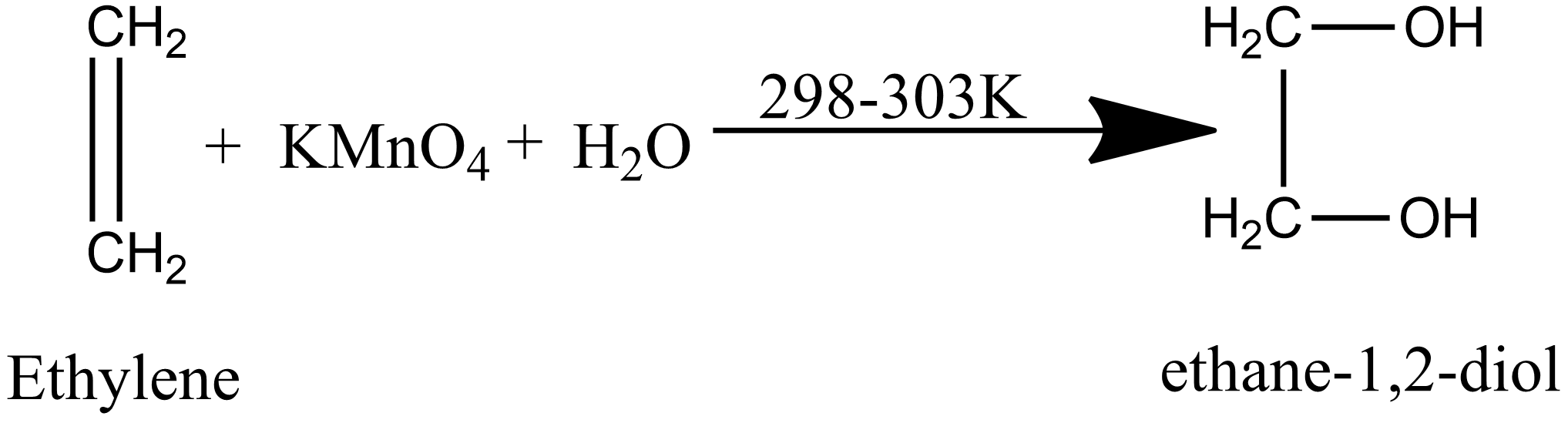
hydroxyl groups across the double bond thus it is known as hydroxylation reaction. Since during the reaction, the pink color of the potassium permanganate is discharged and a brown colored precipitate of manganese dioxide is obtained thus this reaction is used as a test for unsaturation under the name of Baeyer’s test.
So, the correct answer is Option C.
Additional Information:
Functional groups are important for organic compounds as they determine the properties and nature of organic compounds. The set of chemical tests done for the organic compounds to identify the compound is classified as a qualitative test. Unsaturated compounds are the compounds having double or triple bond and the two common test used to find unsaturation of organic compounds are:
Baeyer’s test
Bromine test
Note: We use Baeyer's test for determining the presence of alkenes which are carbon-carbon double bonded compounds or carbon-carbon triple bonded compounds known as alkynes and \[KMn{O_4}\] is known as Baeyer’s reagent.
Complete step by step answer:
Since potassium permanganate is an oxidizing agent it gives oxygen in neutral as well as in an alkaline medium that oxygen obtained is oxidized the alkene into 1,2-diol. When ethylene or ethene reacts with alkaline potassium permanganate it gives ethane-1,2-diol or ethylene glycol.
The reaction of ethylene with alkaline $Kmn{O_4}$ is given as:
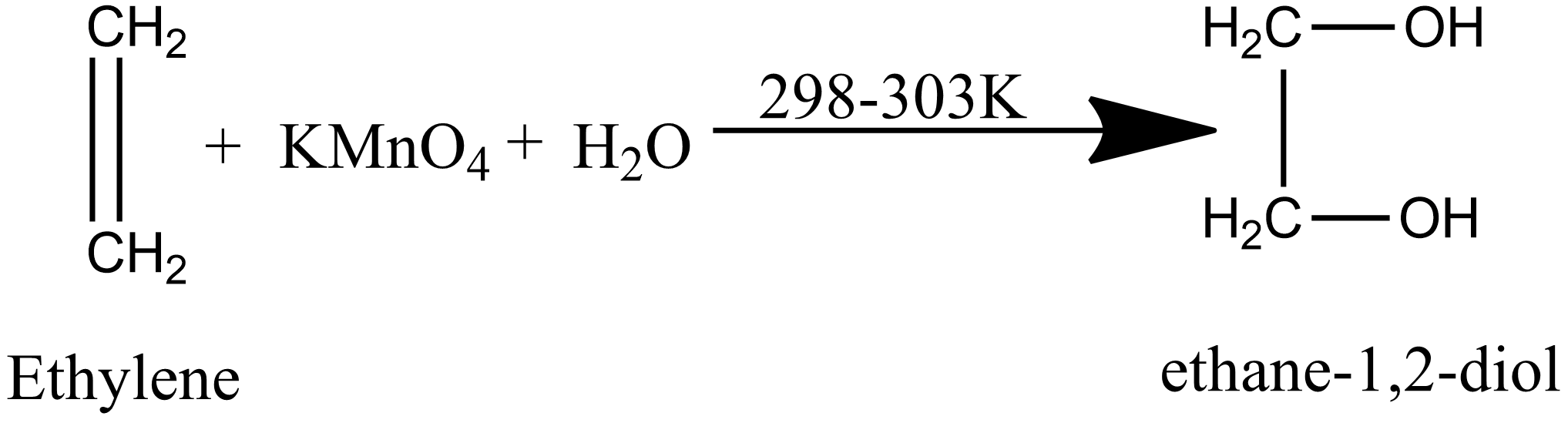
hydroxyl groups across the double bond thus it is known as hydroxylation reaction. Since during the reaction, the pink color of the potassium permanganate is discharged and a brown colored precipitate of manganese dioxide is obtained thus this reaction is used as a test for unsaturation under the name of Baeyer’s test.
So, the correct answer is Option C.
Additional Information:
Functional groups are important for organic compounds as they determine the properties and nature of organic compounds. The set of chemical tests done for the organic compounds to identify the compound is classified as a qualitative test. Unsaturated compounds are the compounds having double or triple bond and the two common test used to find unsaturation of organic compounds are:
Baeyer’s test
Bromine test
Note: We use Baeyer's test for determining the presence of alkenes which are carbon-carbon double bonded compounds or carbon-carbon triple bonded compounds known as alkynes and \[KMn{O_4}\] is known as Baeyer’s reagent.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




