
Ethylidene chloride is a/an:
(A) Vic-dihalide
(B) Gem-dihalide
(C) Allylic halide
(D) Vinylic halide
Answer
585k+ views
Hint: The Ethylidene word in Ethylidene chloride suggests that there are two carbons in which one has one hydrogen and the other has three hydrogens directly bonded to it. It has two carbon atoms in its structure and does not involve a double bond.
Complete step by step solution:
We will first see the structure of the Ethylidene chloride and then we will find which kind of halide it is.
-Ethylidene word is used for the ethane in which one carbon has a hydrogen atom and the other carbon has three hydrogen atoms. So, there should be other two atoms bonded to the carbon which has only one hydrogen atom in order to fulfil its valency of four. So, the structure of Ethylidene chloride can be given as:
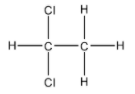
-Now, we will see which type of halide it is.
-Vic-dihalide means vicinal dihalide and this type of dihalide has one halogen atom each on the two adjacent carbon atoms. Ethylidene chloride is not Vic-dihalide,
-Gem-dihalide stands for geminal dihalide and in this type of dihalide, the halogen atoms are bonded with the same carbon atom. So, we can say that Ethylidene chloride is a gem-dihalide.
-Allylic halide is a type of halide in which halogen is at the allylic position of the carbon-carbon double bond.
-Vinylic halide is a type of halide in which halogen is directly bonded to the$s{p^2}$ hybridized carbon of C-C double bond.
-So, we can say that Ethylidene chloride is a Gem-dihalide.
Therefore, the correct answer is (B).
Note: Note that Ethylidene chloride is also known as Ethylidene dichloride as well. So, do not get confused between the two. The IUPAC name of Ethylidene dichloride is 1,1-dichloroethane.
Complete step by step solution:
We will first see the structure of the Ethylidene chloride and then we will find which kind of halide it is.
-Ethylidene word is used for the ethane in which one carbon has a hydrogen atom and the other carbon has three hydrogen atoms. So, there should be other two atoms bonded to the carbon which has only one hydrogen atom in order to fulfil its valency of four. So, the structure of Ethylidene chloride can be given as:

-Now, we will see which type of halide it is.
-Vic-dihalide means vicinal dihalide and this type of dihalide has one halogen atom each on the two adjacent carbon atoms. Ethylidene chloride is not Vic-dihalide,
-Gem-dihalide stands for geminal dihalide and in this type of dihalide, the halogen atoms are bonded with the same carbon atom. So, we can say that Ethylidene chloride is a gem-dihalide.
-Allylic halide is a type of halide in which halogen is at the allylic position of the carbon-carbon double bond.
-Vinylic halide is a type of halide in which halogen is directly bonded to the$s{p^2}$ hybridized carbon of C-C double bond.
-So, we can say that Ethylidene chloride is a Gem-dihalide.
Therefore, the correct answer is (B).
Note: Note that Ethylidene chloride is also known as Ethylidene dichloride as well. So, do not get confused between the two. The IUPAC name of Ethylidene dichloride is 1,1-dichloroethane.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




