
What is evaporation? Explain it on the basis of molecular motion.
Answer
493.2k+ views
Hint: The movement of the constituent particles or molecules in a certain direction is known as molecular motion. There are different types of molecular motions which are translational motion, rotational motion, vibrational motion and electronic motion. The molecular motions of the atoms are affected by the variation of heat and temperature.
Complete answer:
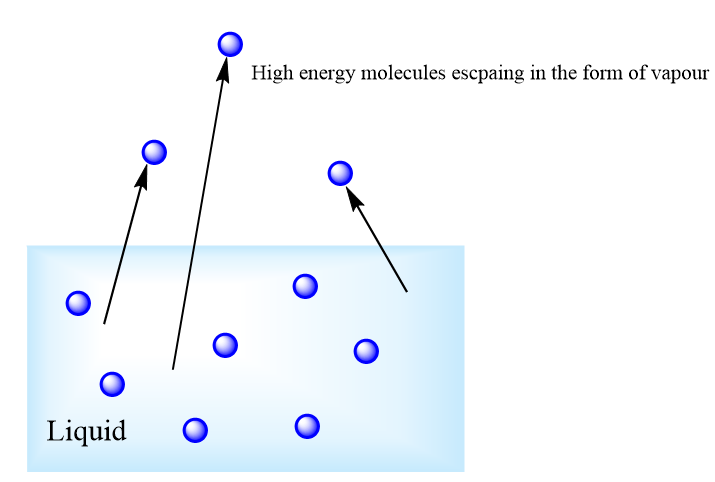
Evaporation: It is the process by which the particles of liquid convert into their vapour phase. In simple words, the change of state of an element from its liquid state to its gaseous state below the temperature at which it boils is known as evaporation.
When a certain liquid is heated, then there is a rapid formation of vapour from all parts of the liquid which is clearly indicated by the visible bubbles. On heating the liquid, that means on increasing the temperature of the liquid, the average kinetic energy of the liquid molecules increases and when the molecules acquire a sufficient amount of kinetic energy, they do work against the force of attraction of the other molecules.
Now, these molecules start leaving the liquid and escape into the open space above it. These escaping molecules form the vapour of the liquid and the process continues till all the liquid molecules evaporates.
Note:
It is important to note that evaporation takes place at all the temperatures, but the vaporization process takes place only at a fixed temperature which is known as the boiling point of the liquid.
Complete answer:
Evaporation: It is the process by which the particles of liquid convert into their vapour phase. In simple words, the change of state of an element from its liquid state to its gaseous state below the temperature at which it boils is known as evaporation.
When a certain liquid is heated, then there is a rapid formation of vapour from all parts of the liquid which is clearly indicated by the visible bubbles. On heating the liquid, that means on increasing the temperature of the liquid, the average kinetic energy of the liquid molecules increases and when the molecules acquire a sufficient amount of kinetic energy, they do work against the force of attraction of the other molecules.
Now, these molecules start leaving the liquid and escape into the open space above it. These escaping molecules form the vapour of the liquid and the process continues till all the liquid molecules evaporates.

Note:
It is important to note that evaporation takes place at all the temperatures, but the vaporization process takes place only at a fixed temperature which is known as the boiling point of the liquid.
Recently Updated Pages
Master Class 8 Social Science: Engaging Questions & Answers for Success

Master Class 8 English: Engaging Questions & Answers for Success

Class 8 Question and Answer - Your Ultimate Solutions Guide

Master Class 8 Maths: Engaging Questions & Answers for Success

Master Class 8 Science: Engaging Questions & Answers for Success

Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Trending doubts
What is BLO What is the full form of BLO class 8 social science CBSE

Citizens of India can vote at the age of A 18 years class 8 social science CBSE

Full form of STD, ISD and PCO

Advantages and disadvantages of science

Right to vote is a AFundamental Right BFundamental class 8 social science CBSE

What are the 12 elements of nature class 8 chemistry CBSE




