
What is an example of a tetrahedral bent molecule other than water?
Answer
528k+ views
Hint: The shape or geometry of molecules is a result of the presence of bond pairs and lone pairs in that molecule. The lone pairs of electrons acquire a place in the molecule to minimize repulsion and therefore they distort the geometry of the molecule.
Complete answer:
Water has its chemical name as hydrogen oxide. It has 2 bond pairs and 2 lone pairs of electrons that gives it a tetrahedral bent shape. The lone pairs occupy such a position so that they can minimize repulsion, hence the geometry is tetrahedral.
Water molecule has oxygen as the central atom, similarly another molecule, like water, is ${{H}_{2}}S$. As oxygen and sulphur both are from group 16, they tend to form hydrides with hydrogen with 2 bonds and 2 lone pairs of electrons. So, ${{H}_{2}}S$ molecule also has the same geometry as that of water molecule. The geometry of ${{H}_{2}}S$ is:
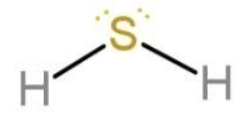
The difference is only in the bond lengths. Sulphur and hydrogen bond length is longer in ${{H}_{2}}S$ (133.6 pm), while oxygen and hydrogen bond length is shorter in ${{H}_{2}}O$(95.8 pm). This is due to the fact that the size of atoms increases down the group, due to increase in the valence shells.
Hence, ${{H}_{2}}S$ molecule is a tetrahedral bent molecule other than water.
Note:
These geometries of molecules are according to the VSEPR. This theory suggests that the shape of any molecule depends on a bond and lone pair of electrons. The repulsive interactions are in the order, lone pair – lone pair repulsion > lone pair – bond pair repulsion > bond pair – bond pair repulsion.
Complete answer:
Water has its chemical name as hydrogen oxide. It has 2 bond pairs and 2 lone pairs of electrons that gives it a tetrahedral bent shape. The lone pairs occupy such a position so that they can minimize repulsion, hence the geometry is tetrahedral.
Water molecule has oxygen as the central atom, similarly another molecule, like water, is ${{H}_{2}}S$. As oxygen and sulphur both are from group 16, they tend to form hydrides with hydrogen with 2 bonds and 2 lone pairs of electrons. So, ${{H}_{2}}S$ molecule also has the same geometry as that of water molecule. The geometry of ${{H}_{2}}S$ is:

The difference is only in the bond lengths. Sulphur and hydrogen bond length is longer in ${{H}_{2}}S$ (133.6 pm), while oxygen and hydrogen bond length is shorter in ${{H}_{2}}O$(95.8 pm). This is due to the fact that the size of atoms increases down the group, due to increase in the valence shells.
Hence, ${{H}_{2}}S$ molecule is a tetrahedral bent molecule other than water.
Note:
These geometries of molecules are according to the VSEPR. This theory suggests that the shape of any molecule depends on a bond and lone pair of electrons. The repulsive interactions are in the order, lone pair – lone pair repulsion > lone pair – bond pair repulsion > bond pair – bond pair repulsion.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




