
Expanded octet can be observed in the valence shell of the central atom in:
A. $N{H_3}$
B. $C{H_4}$
C. $PC{l_5}$
D. $BeCl_2$
Answer
569.4k+ views
Hint: We can say transfer of electrons as a process where an electron shares one or more electrons to its nearby atom. We know that there must be eight electrons in the outermost orbital of an atom. This is known as the octet rule. If an atom contains less than eight electrons, they have the ability to react and produce stable compounds.
Complete step by step answer:
We have to remember that the $N{H_3}$ has six electrons in its bond pair and there is one lone pair (two electrons) present in nitrogen, so a sum of eight electrons are obtained. We can draw the structure of this compound as,
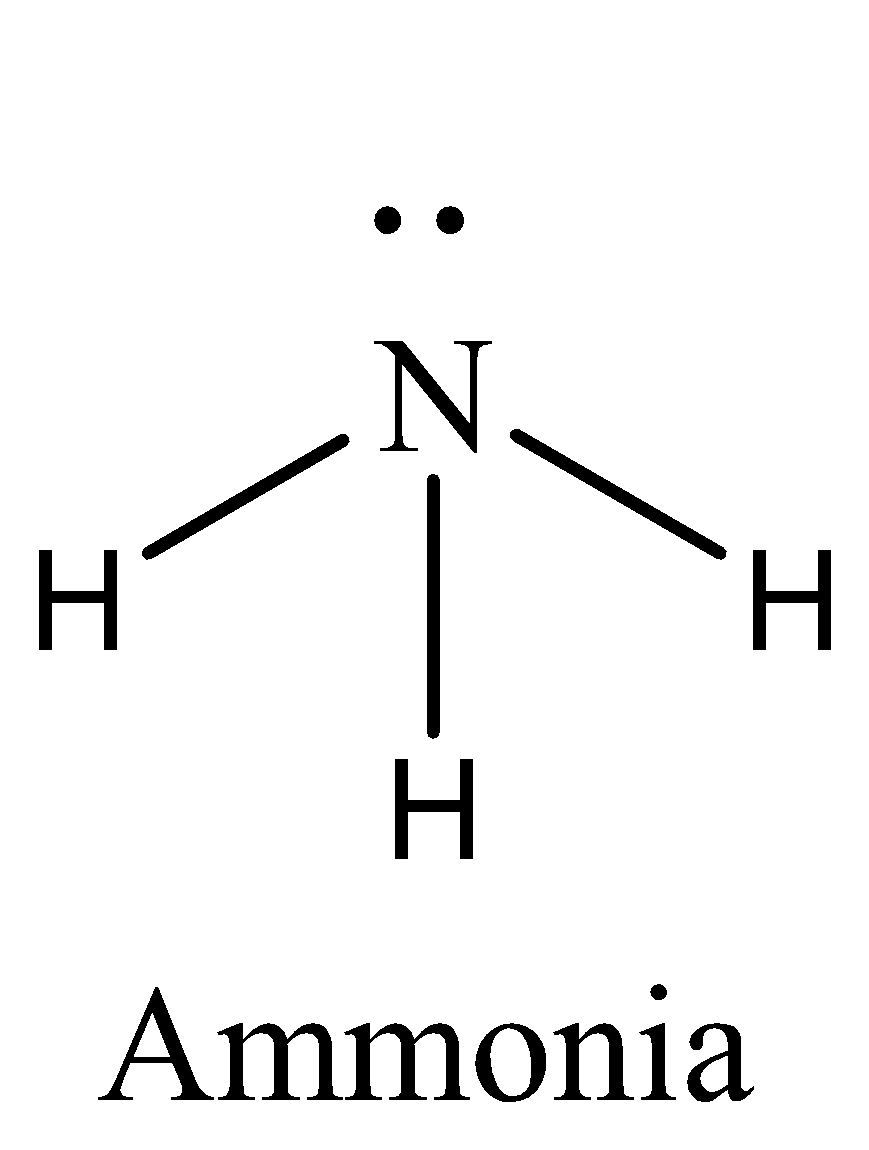
So $N{H_3}$ does not have an expanded octet. Therefore, the option (A) is incorrect.
As we know that the $C{H_4}$ has eight electrons in its bond pair and there is no one lone pair present in carbon, so a total of eight electrons are obtained. We can draw the structure of this compound as,
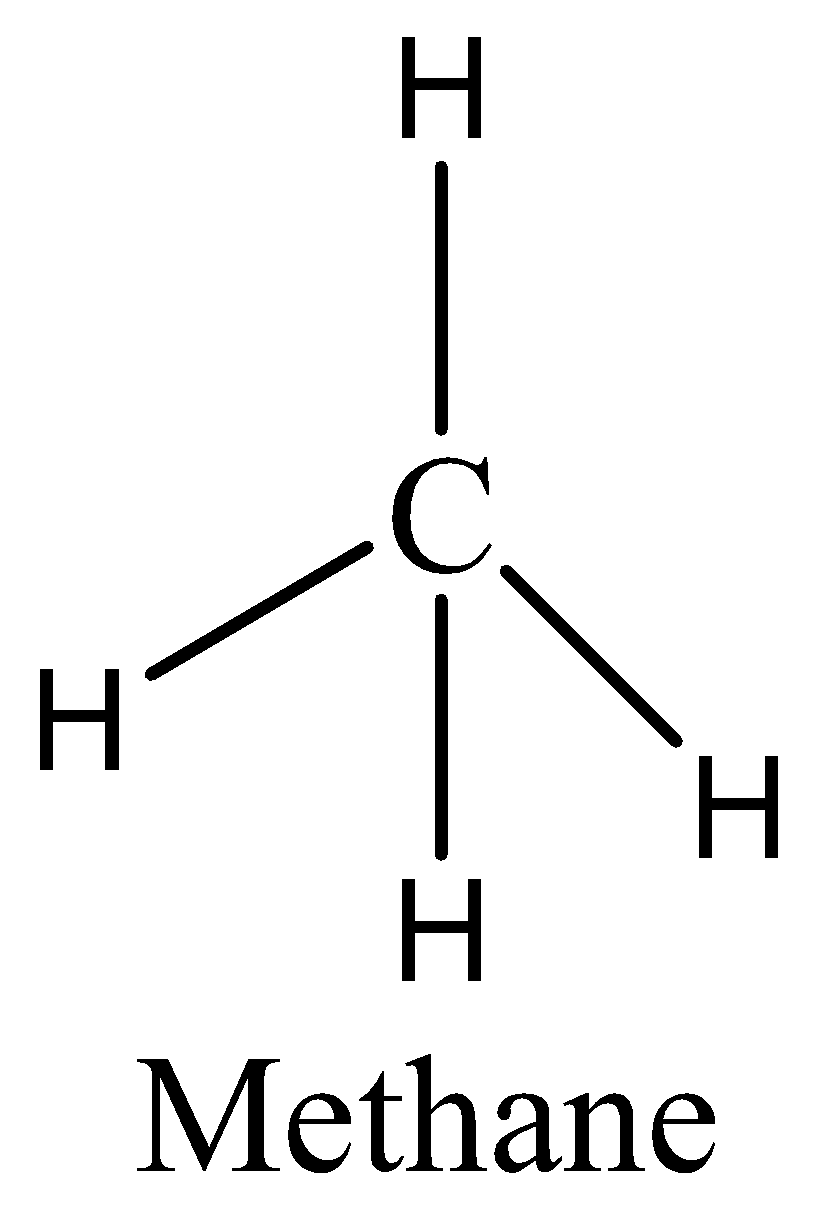
So $C{H_4}$ does not have an expanded octet. Therefore, the option (B) is incorrect.
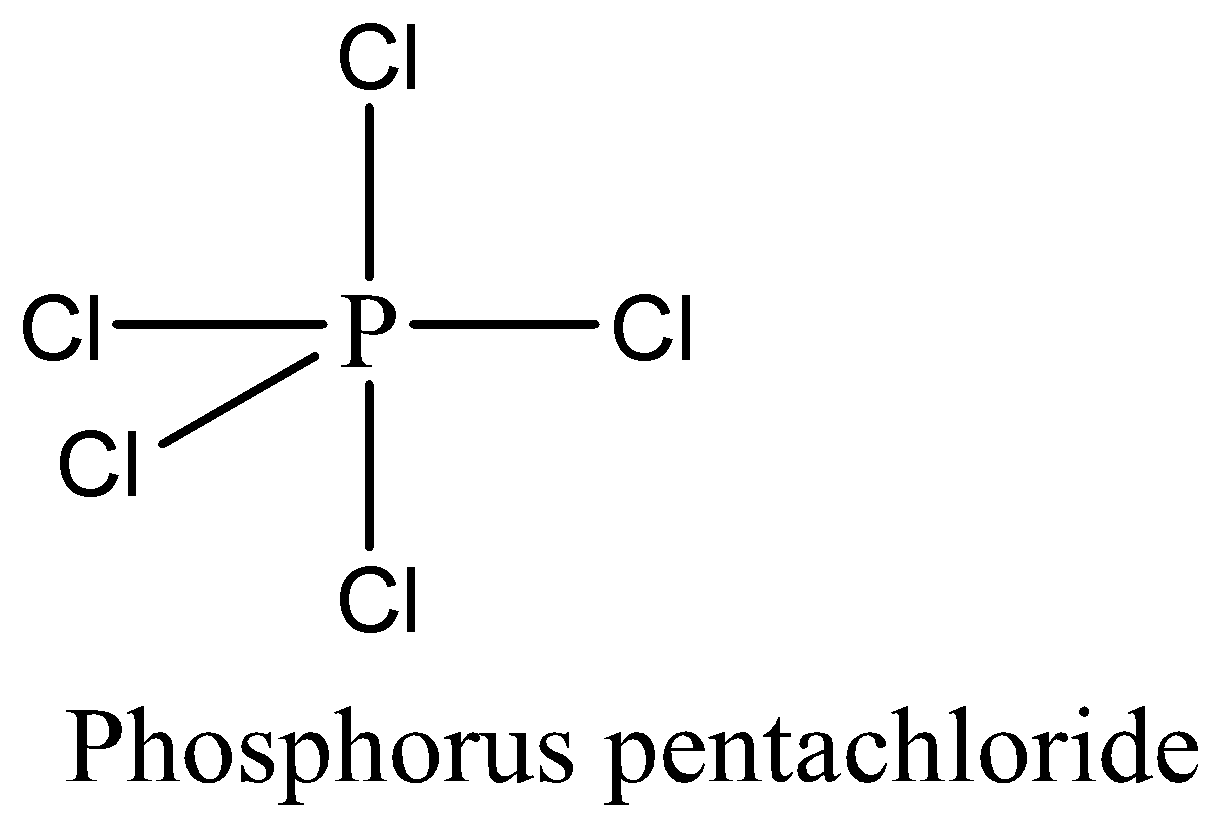
We have to remember that the $PC{l_5}$ has 10 numbers of bonding electrons by sharing 5 valence electrons of phosphorus with 5 chlorine atoms and no lone pair of electrons are present. The expanded octet rule is satisfied by $PC{l_5}$.
Therefore, the option (C) is correct. We can draw the structure of this compound as,
We have to remember that the $BeC{l_2}$ does not show expanded octet, beryllium atoms join with each atom of chlorine through a single bond. Instead of an octet, the outermost shell of beryllium has two electrons pairs. They do not have any complete octet.
Therefore, the option (D) is incorrect. We can draw the structure of this compound as,
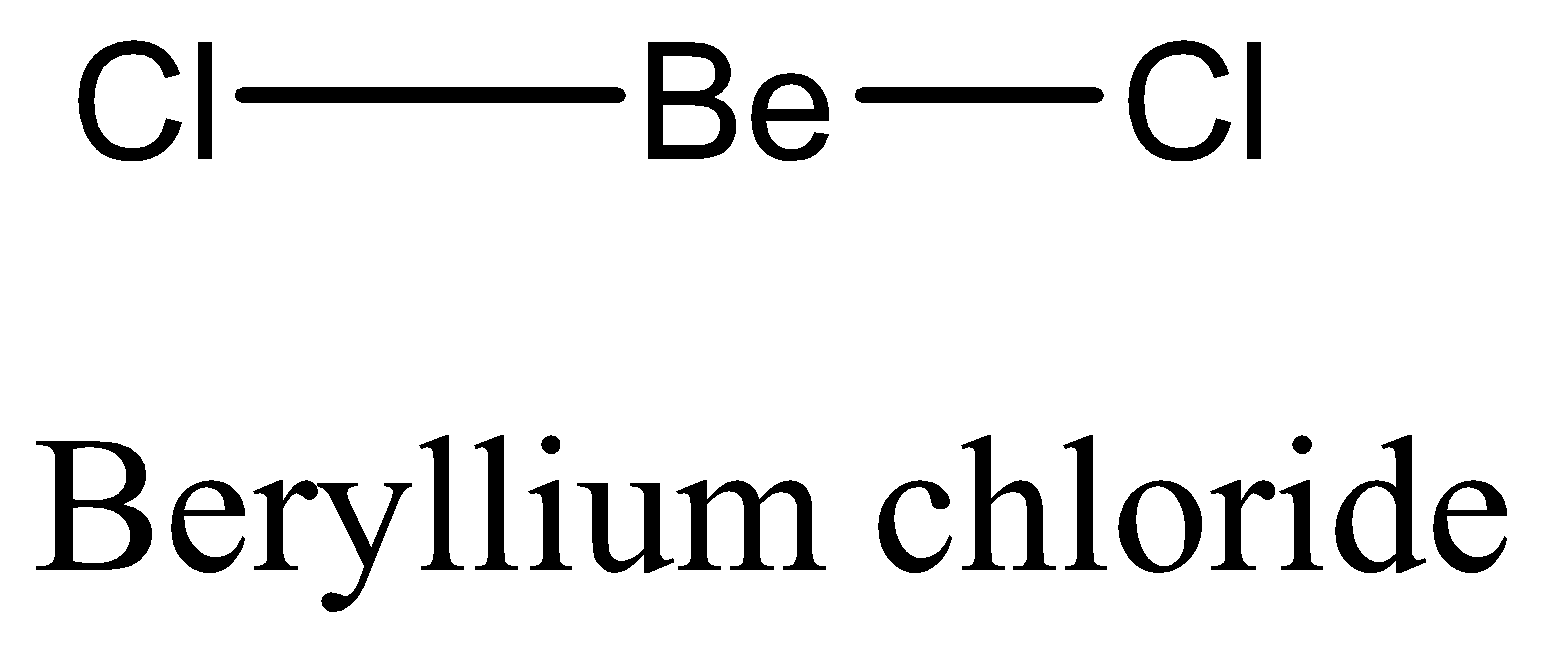
So, the correct answer is Option C.
Note: We have to remember that in $S{F_6}$ the atom that does not obey octet rule is sulfur. The central sulfur atom gives six covalent bonds to six fluorine atoms. So it is an expanded valence shell molecule. The atom of sulfur expands its octet, hence the molecule $S{F_6}$ interrupts the octet rule. In $B{H_3}$ the atom which differs from the octet rule is boron. There are only six valence electrons in $B{H_3}$ around the central atom boron. The atom of boron exhibits incomplete octet and so, the molecule $B{H_3}$ disturbs the octet rule.
Complete step by step answer:
We have to remember that the $N{H_3}$ has six electrons in its bond pair and there is one lone pair (two electrons) present in nitrogen, so a sum of eight electrons are obtained. We can draw the structure of this compound as,

So $N{H_3}$ does not have an expanded octet. Therefore, the option (A) is incorrect.
As we know that the $C{H_4}$ has eight electrons in its bond pair and there is no one lone pair present in carbon, so a total of eight electrons are obtained. We can draw the structure of this compound as,

So $C{H_4}$ does not have an expanded octet. Therefore, the option (B) is incorrect.
We have to remember that the $PC{l_5}$ has 10 numbers of bonding electrons by sharing 5 valence electrons of phosphorus with 5 chlorine atoms and no lone pair of electrons are present. The expanded octet rule is satisfied by $PC{l_5}$.
Therefore, the option (C) is correct. We can draw the structure of this compound as,

We have to remember that the $BeC{l_2}$ does not show expanded octet, beryllium atoms join with each atom of chlorine through a single bond. Instead of an octet, the outermost shell of beryllium has two electrons pairs. They do not have any complete octet.
Therefore, the option (D) is incorrect. We can draw the structure of this compound as,
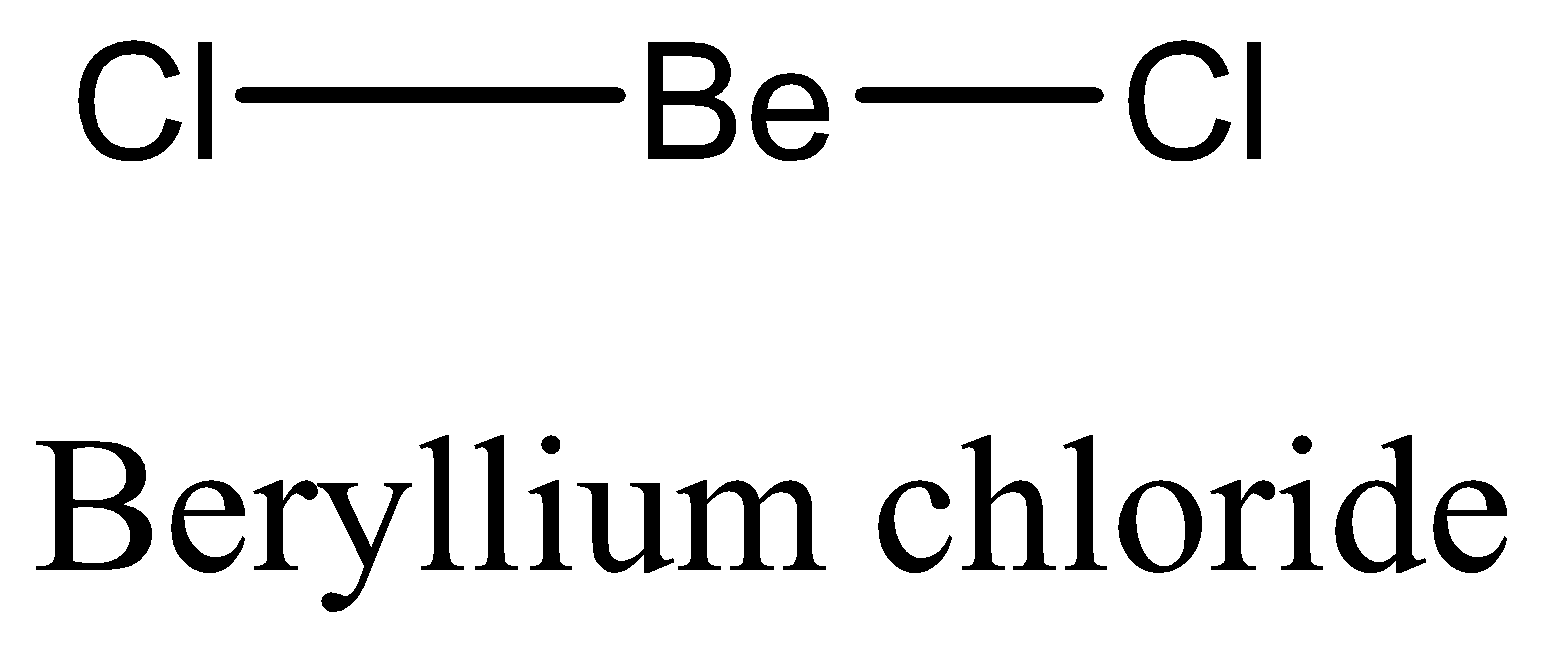
So, the correct answer is Option C.
Note: We have to remember that in $S{F_6}$ the atom that does not obey octet rule is sulfur. The central sulfur atom gives six covalent bonds to six fluorine atoms. So it is an expanded valence shell molecule. The atom of sulfur expands its octet, hence the molecule $S{F_6}$ interrupts the octet rule. In $B{H_3}$ the atom which differs from the octet rule is boron. There are only six valence electrons in $B{H_3}$ around the central atom boron. The atom of boron exhibits incomplete octet and so, the molecule $B{H_3}$ disturbs the octet rule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




