
Explain a free expansion process with an example.
Answer
537.6k+ views
Hint: The term expansions means moving out from a smaller area to a larger area. There is also the term ‘free’ associated with it, free can mean that a gas is free to expand on its own.
Complete answer:
To explain what is free expansion is, consider two similar boxes connected through an insulated pipe like structure, we can call the first box as box A and second box B. The box A has a gas of pressure P and volume V and at temperature T. The box B is completely in vacuum and there is a plug like substance (rubber cork) in the connection which prevents the gas from box A from entering box B.
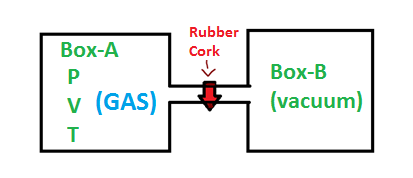
When the plug is removed, the gas from box A will start to occupy box B without any external pressure. This expansion process in which the gas expands freely from one box to another is called free expansion.
When a free expansion takes place, there is no external pressure acting on the gas, which makes the gas expand. The gas expands on its own without external pressure acting on it. So according to the equation of work in thermodynamics $\text{W}={{\text{P}}_{\text{ext}}}\text{ }\!\!\Delta\!\!\text{ V}$.
Note:
There is also no change in internal energy since the temperature remains constant throughout the process.
Also according to the first law of thermodynamics, when work done and change in internal energy is zero, the heat released or absorbed is zero. $\text{ }\!\!\Delta\!\!\text{ Q}=\text{ }\!\!\Delta\!\!\text{ U}+\text{W}$, so in this process $\text{ }\!\!\Delta\!\!\text{ U and W}$ is zero. So $\text{ }\!\!\Delta\!\!\text{ Q}$ is zero.
Complete answer:
To explain what is free expansion is, consider two similar boxes connected through an insulated pipe like structure, we can call the first box as box A and second box B. The box A has a gas of pressure P and volume V and at temperature T. The box B is completely in vacuum and there is a plug like substance (rubber cork) in the connection which prevents the gas from box A from entering box B.

When the plug is removed, the gas from box A will start to occupy box B without any external pressure. This expansion process in which the gas expands freely from one box to another is called free expansion.
When a free expansion takes place, there is no external pressure acting on the gas, which makes the gas expand. The gas expands on its own without external pressure acting on it. So according to the equation of work in thermodynamics $\text{W}={{\text{P}}_{\text{ext}}}\text{ }\!\!\Delta\!\!\text{ V}$.
Note:
There is also no change in internal energy since the temperature remains constant throughout the process.
Also according to the first law of thermodynamics, when work done and change in internal energy is zero, the heat released or absorbed is zero. $\text{ }\!\!\Delta\!\!\text{ Q}=\text{ }\!\!\Delta\!\!\text{ U}+\text{W}$, so in this process $\text{ }\!\!\Delta\!\!\text{ U and W}$ is zero. So $\text{ }\!\!\Delta\!\!\text{ Q}$ is zero.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




