
Explain Endothermic Reaction with examples?
Answer
590.4k+ views
Hint: The system absorbs heat from the surroundings, the entropy of the surrounding decreases & the enthalpy change is positive.
Complete step by step answer:
Endothermic reactions are chemical reactions in which the reactants absorb heat energy from surroundings to from products. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. Physical heat energy from their surroundings and melt to form liquid water (no chemical bonds are broken or formed) when a chemical bond is broken, it is usually accompanied by a release of energy. Similarly the formation of chemical bonds requires an input of energy. The energy supplied can be of various farms (such as heat, light & electricity). Endothermic reactions generally involve the formation of chemical bonds through the absorption of heat from the surroundings. On the other band exothermic reactions involve the release of heat energy generated from bond – breakage.
Examples:
-When ammonium chloride (NH4 Cl) is dissolved is water an Endothermic reaction takes place. The salt dissociates into ammonium (NH+4) and chloride (cl-) ions.
\[\text{N}{{\text{H}}_{\text{4}}}\text{Cl(S)}\,+\,{{\text{H}}_{\text{2}}}\text{O}\,(\text{i})\,\to \,\text{N}{{\text{H}}_{4}}\text{Cl}(\text{aq})-\text{Heat}\]
-Ammonium nitrate $(\text{N}{{\text{H}}_{4}}\text{N}{{\text{O}}_{3}})$ as important component is instant cold packs, dissociate is instant cold packs, dissociate into the ammonium cation $(\text{NH}_{4}^{+})$ and the nitrate ion $(\text{NO}_{3}^{-})$ when dissolved in water. These ions go on to form ammonium hydroxide and nitric acid respectively reacting with OH- and H+ ions is water.
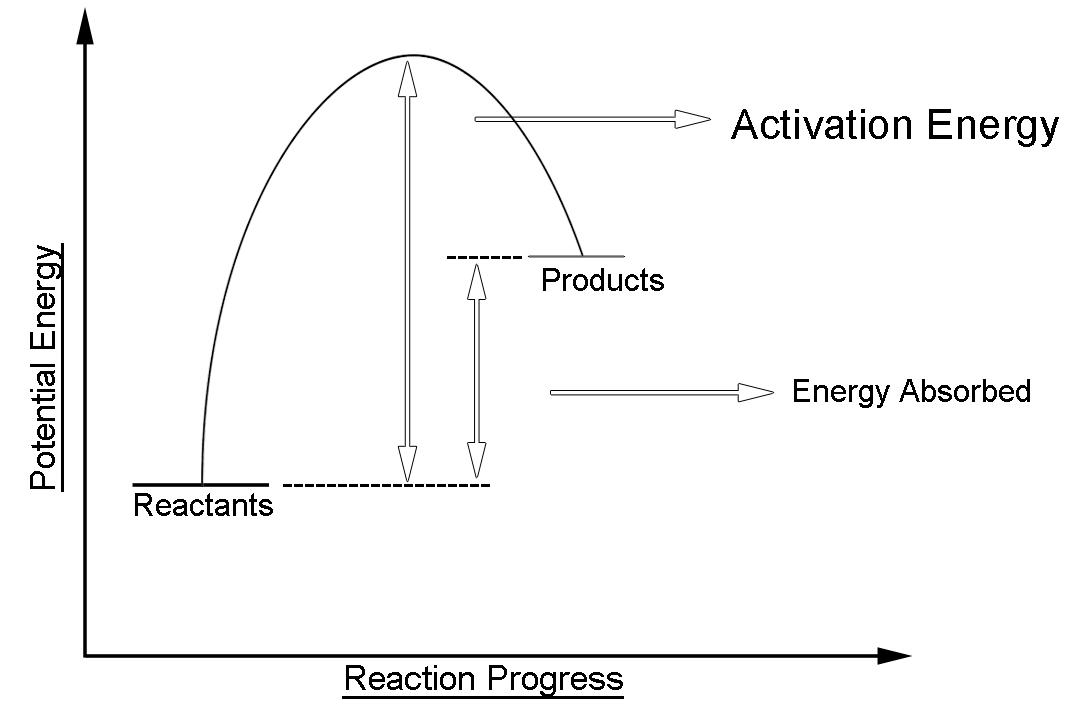
-The simple energy level diagram of endothermic reaction is the activation energy that must be provided to the reactants so that they can overcome the energy barrier and react.
Note:
The human body exploits the endothermic nature of evaporation to cool itself. This is done through the process of sweating. The potential energy of the product is an Endothermic reaction is higher than that of reactants. This gap is the potential energy that accounts for the energy that was absorbed by the system during the chemical reaction.
Complete step by step answer:
Endothermic reactions are chemical reactions in which the reactants absorb heat energy from surroundings to from products. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. Physical heat energy from their surroundings and melt to form liquid water (no chemical bonds are broken or formed) when a chemical bond is broken, it is usually accompanied by a release of energy. Similarly the formation of chemical bonds requires an input of energy. The energy supplied can be of various farms (such as heat, light & electricity). Endothermic reactions generally involve the formation of chemical bonds through the absorption of heat from the surroundings. On the other band exothermic reactions involve the release of heat energy generated from bond – breakage.
Examples:
-When ammonium chloride (NH4 Cl) is dissolved is water an Endothermic reaction takes place. The salt dissociates into ammonium (NH+4) and chloride (cl-) ions.
\[\text{N}{{\text{H}}_{\text{4}}}\text{Cl(S)}\,+\,{{\text{H}}_{\text{2}}}\text{O}\,(\text{i})\,\to \,\text{N}{{\text{H}}_{4}}\text{Cl}(\text{aq})-\text{Heat}\]
-Ammonium nitrate $(\text{N}{{\text{H}}_{4}}\text{N}{{\text{O}}_{3}})$ as important component is instant cold packs, dissociate is instant cold packs, dissociate into the ammonium cation $(\text{NH}_{4}^{+})$ and the nitrate ion $(\text{NO}_{3}^{-})$ when dissolved in water. These ions go on to form ammonium hydroxide and nitric acid respectively reacting with OH- and H+ ions is water.
-The simple energy level diagram of endothermic reaction is the activation energy that must be provided to the reactants so that they can overcome the energy barrier and react.

Note:
The human body exploits the endothermic nature of evaporation to cool itself. This is done through the process of sweating. The potential energy of the product is an Endothermic reaction is higher than that of reactants. This gap is the potential energy that accounts for the energy that was absorbed by the system during the chemical reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




