
Explain Fractional distillation of petroleum.
Answer
586.2k+ views
Hint: Separating petroleum into useful fractions and removal of impurities is called refining. The refining of petroleum is done by a process called fractional distillation.
Complete step by step answer:
So, generally fractional distillation is a special type of distillation designed to separate a mixture of two or more liquids which tend to have different boiling points.
- The process involves heating the mixture and partial condensation of the vapors along a fractionating column which is set up such that components with lower boiling points pass through the column and which are collected before the components with higher boiling point.
- Generally, the crude petroleum obtained is a dark viscous liquid which contains many impurities, in order to make it useful it must be separated into various components. The refining of petroleum is done by a process called fractional distillation.
Let us know the process step by step.
- In the first process neutralization of crude oil takes place, this is nothing but the refining process here, crude oil will be washed with acidic or basic solution as needed.
- The oil is heated in a furnace about 675K and the vapors are induced into the fractionating tower. The tower consists of holes and shelves and these holes have bubble caps which allow only lighter components to escape while the heavier components flow into trays below.
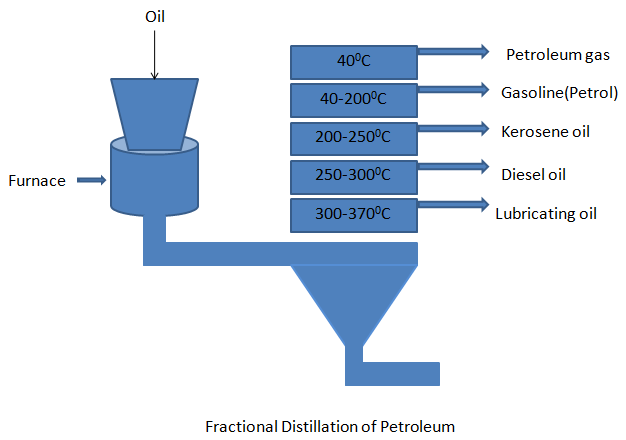
- Therefore, fractions with lower boiling points rise up the tower and condense at different levels according to their boiling points.
- The components with the highest boiling point liquefies first and are collected, the components having lower boiling point liquefies so on.
Note: This process is also used for the purification of water as well as separating acetone and water. You should know that fractional distillation is important as it can be used to isolate a specific chemical species for further analysis.
Complete step by step answer:
So, generally fractional distillation is a special type of distillation designed to separate a mixture of two or more liquids which tend to have different boiling points.
- The process involves heating the mixture and partial condensation of the vapors along a fractionating column which is set up such that components with lower boiling points pass through the column and which are collected before the components with higher boiling point.
- Generally, the crude petroleum obtained is a dark viscous liquid which contains many impurities, in order to make it useful it must be separated into various components. The refining of petroleum is done by a process called fractional distillation.
Let us know the process step by step.
- In the first process neutralization of crude oil takes place, this is nothing but the refining process here, crude oil will be washed with acidic or basic solution as needed.
- The oil is heated in a furnace about 675K and the vapors are induced into the fractionating tower. The tower consists of holes and shelves and these holes have bubble caps which allow only lighter components to escape while the heavier components flow into trays below.

- Therefore, fractions with lower boiling points rise up the tower and condense at different levels according to their boiling points.
- The components with the highest boiling point liquefies first and are collected, the components having lower boiling point liquefies so on.
Note: This process is also used for the purification of water as well as separating acetone and water. You should know that fractional distillation is important as it can be used to isolate a specific chemical species for further analysis.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




