
Explain how Hope’s apparatus can be used to demonstrate the anomalous behavior of water?
Answer
526.7k+ views
Hint: Hope’s apparatus is a vertical vessel containing water surrounded around the middle with a trough which is filled with ice. Two thermometers are placed above and below the trough to measure the temperature of the water as shown below:
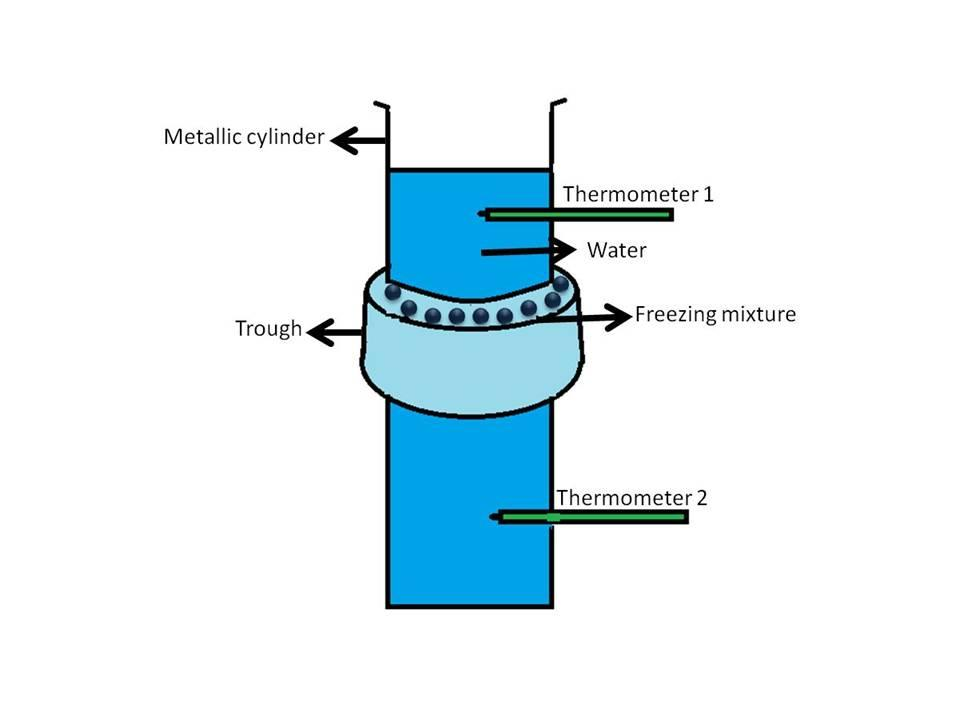
Complete answer:We all know that the density of solids is greater than liquids so it is expected that when a liquid becomes solid its density increases and it contracts. But water on cooling shows contraction till $4^oC$ and after that expands and converts into ice which has a lower density than water (clear from the fact that ice floats on water). This anomalous (different) behavior of water can be demonstrated by the use of an instrument called Hope’s apparatus. In this experiment the temperature of the water-filled in a cylinder vessel is varied. It is conducted in two sets:
$1.$ In the first set, the water is maintained at a temperature higher than $4^oC$, and the cylindrical vessel is filled with this water. The reading of the two thermometers is noted (it is observed to be the same in the beginning). Then freezing mixture containing ice and salt id added to the trough and after some time (say $30$ minutes) temperature is noted. In this case, the temperature of the water in the lower part of the vessel below the trough is found to be less as compared to the upper part. It can be explained by keeping in mind that the addition of freezing mixture decreased the temperature of the water and due to an increase in density (above $4^oC$) the water at lower temperature sinks down resulting in a lowering of the temperature of the thermometer $2$ as compared to the temperature of thermometer $1$.
$2.$ In the second set, the water is maintained to a temperature near to $4^oC$ and filled in the cylinder. After the addition of the freezing mixture, the temperature of water drops below $4^oC$ and it begins to expand thus comes to the upper part (remember, ice floats on water). Thus, the temperature of the upper part of the vessel decreases resulting in a lowering of the temperature of the thermometer $1$ as compared to the temperature of the thermometer $2$.
Note:
During the experiment, the temperature of the water should vary between $0^oC$ to higher than $4^oC$ otherwise the change will not be observed. Both water to be filled in the vessel and the apparatus itself is maintained at the same temperature.

Complete answer:We all know that the density of solids is greater than liquids so it is expected that when a liquid becomes solid its density increases and it contracts. But water on cooling shows contraction till $4^oC$ and after that expands and converts into ice which has a lower density than water (clear from the fact that ice floats on water). This anomalous (different) behavior of water can be demonstrated by the use of an instrument called Hope’s apparatus. In this experiment the temperature of the water-filled in a cylinder vessel is varied. It is conducted in two sets:
$1.$ In the first set, the water is maintained at a temperature higher than $4^oC$, and the cylindrical vessel is filled with this water. The reading of the two thermometers is noted (it is observed to be the same in the beginning). Then freezing mixture containing ice and salt id added to the trough and after some time (say $30$ minutes) temperature is noted. In this case, the temperature of the water in the lower part of the vessel below the trough is found to be less as compared to the upper part. It can be explained by keeping in mind that the addition of freezing mixture decreased the temperature of the water and due to an increase in density (above $4^oC$) the water at lower temperature sinks down resulting in a lowering of the temperature of the thermometer $2$ as compared to the temperature of thermometer $1$.
$2.$ In the second set, the water is maintained to a temperature near to $4^oC$ and filled in the cylinder. After the addition of the freezing mixture, the temperature of water drops below $4^oC$ and it begins to expand thus comes to the upper part (remember, ice floats on water). Thus, the temperature of the upper part of the vessel decreases resulting in a lowering of the temperature of the thermometer $1$ as compared to the temperature of the thermometer $2$.
Note:
During the experiment, the temperature of the water should vary between $0^oC$ to higher than $4^oC$ otherwise the change will not be observed. Both water to be filled in the vessel and the apparatus itself is maintained at the same temperature.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




