
Explain hybridization of central atoms in \[CC{l_4}\].
Answer
600.3k+ views
Hint: There are mainly three types of orbital hybridization in chemistry: ${ sp }^{ 3 }$, ${ sp }^{ 2 }$, and sp. The state of hybridization of an atom can be determined from the steric number of that atom. The steric number is calculated from the Lewis structure of the respective molecule.
Complete answer:
Carbon tetrachloride is a liquid organic compound that has a molecular formula \[CC{l_4}\]. It is widely used as a solvent in various organic chemical reactions. The central atom of this molecule is obviously carbon (C) and four chlorine atoms are attached to it by four σ-bonds.
The state of hybridization of an atom in a particular molecule can be obtained by determining the steric number (SN) of that atom.
The steric number is the summation of the number of lone pairs of electrons present on the atom and the number of atoms that are directly attached to that. Mathematically, it can be represented as:
SN = (lone pairs of electrons on the atom) + (number of atoms directly bonded to the atom)
Now, the steric number is related to the state of hybridization is as follows:
If the steric number is 2, then the hybridization is sp.
If the steric number is 3, then the hybridization is ${ sp }^{ 2 }$.
If the steric number is 4, then the hybridization is ${ sp }^{ 3 }$.
Now, the steric number can be evaluated from the Lewis structure of a molecule.
The Lewis structure of \[CC{l_4}\]is given below:
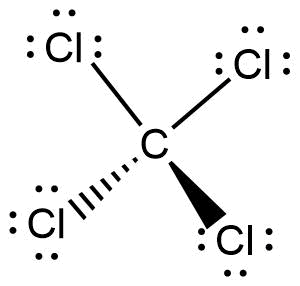
From the above structure, it can be concluded that,
Lone pairs on carbon atom is 0
Number of atoms that are bonded to carbon atom is 4
Hence, the steric number will be:
SN = (0+4) = 4
Since, the steric number of carbon atoms is found to be four, then we can say that the state of hybridization of carbon atoms is ${ sp }^{ 3 }$.
Hence, the hybridization of the central atom in \[CC{l_4}\] is ${ sp }^{ 3 }$.
Note: The shape and geometry of a molecule depends upon the hybridization state of the central atom of a molecule. If the central atom is Sp3 hybridized, then both the geometry and the shape of the molecule will be tetrahedral.
Complete answer:
Carbon tetrachloride is a liquid organic compound that has a molecular formula \[CC{l_4}\]. It is widely used as a solvent in various organic chemical reactions. The central atom of this molecule is obviously carbon (C) and four chlorine atoms are attached to it by four σ-bonds.
The state of hybridization of an atom in a particular molecule can be obtained by determining the steric number (SN) of that atom.
The steric number is the summation of the number of lone pairs of electrons present on the atom and the number of atoms that are directly attached to that. Mathematically, it can be represented as:
SN = (lone pairs of electrons on the atom) + (number of atoms directly bonded to the atom)
Now, the steric number is related to the state of hybridization is as follows:
If the steric number is 2, then the hybridization is sp.
If the steric number is 3, then the hybridization is ${ sp }^{ 2 }$.
If the steric number is 4, then the hybridization is ${ sp }^{ 3 }$.
Now, the steric number can be evaluated from the Lewis structure of a molecule.
The Lewis structure of \[CC{l_4}\]is given below:

From the above structure, it can be concluded that,
Lone pairs on carbon atom is 0
Number of atoms that are bonded to carbon atom is 4
Hence, the steric number will be:
SN = (0+4) = 4
Since, the steric number of carbon atoms is found to be four, then we can say that the state of hybridization of carbon atoms is ${ sp }^{ 3 }$.
Hence, the hybridization of the central atom in \[CC{l_4}\] is ${ sp }^{ 3 }$.
Note: The shape and geometry of a molecule depends upon the hybridization state of the central atom of a molecule. If the central atom is Sp3 hybridized, then both the geometry and the shape of the molecule will be tetrahedral.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




