
Explain isolation method to determine rate law and order of reaction.
Answer
592.2k+ views
Hint: There are few methods of determining the rate law of a reaction. The isolation method is also a method of determining the rate law of a reaction. Try to figure out what isolation means in this context. Think about the isolated rate law of each of the reactants.
Complete step by step answer: We know that the rate law of a reaction can be determined experimentally by isolation method and some other methods. In the isolation method, we isolate one of the reactants each time keeping other reactant concentrations constant. This experiment is repeated by isolating each reactant at a time.
Consider a reaction: $aA + bB \rightarrow cC + dD $
Let us calculate the rate law for this reaction. There are 2 reactant species so we need to perform reaction 2 times isolating each reactant.
First, let us isolate A and calculate order with respect to A by keeping the concentration of B constant. Let the order of reaction with respect to A be x. Then we will get the rate of the reaction as
Rate r = ${ k }_{ 1 }\left[ A \right] ^{ x }$
Let $\left[ { B }_{ 0 } \right]$ be the constant concentration of B.
${ k }_{ 1 } = { k }\left[ { B }_{ 0 } \right] ^{ y }$ where y is the order of reaction with respect to B.
Applying log to rate expression
\[\log { \left( r \right) } =\log { \left( k \right) + } x\log { \left( \left[ A \right] \right) }\]
We plot the above equation with log r on the y-axis and log [A] values on the x-axis. We find the slope of the straight line which is nothing but the order of reaction with respect to A.
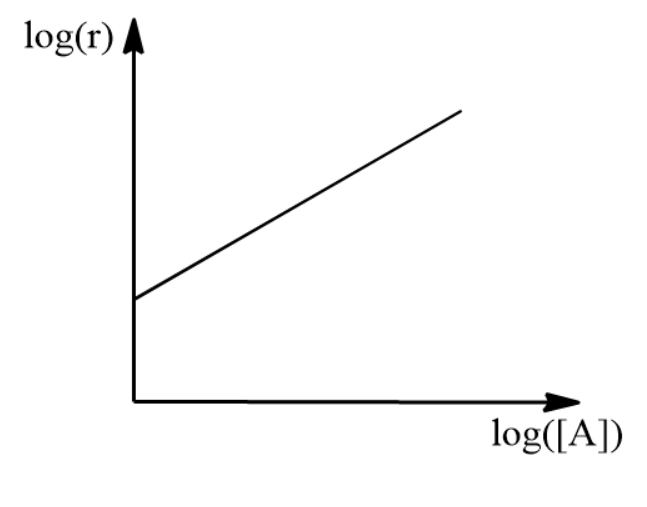
Repeating the above procedure by keeping concentration A constant we get the order with respect to B. Combining these two orders we get the rate law of reaction. Adding orders of reaction with respect to each reactant we can get the total order of the reaction.
Note: This method is easy as we require less calculation to perform at a time. We can keep the reactant’s concentration common by taking that reactant concentration very large. As we take concentration large there will not be much change in the concentration of that reactant. So, it will nearly be constant.
Complete step by step answer: We know that the rate law of a reaction can be determined experimentally by isolation method and some other methods. In the isolation method, we isolate one of the reactants each time keeping other reactant concentrations constant. This experiment is repeated by isolating each reactant at a time.
Consider a reaction: $aA + bB \rightarrow cC + dD $
Let us calculate the rate law for this reaction. There are 2 reactant species so we need to perform reaction 2 times isolating each reactant.
First, let us isolate A and calculate order with respect to A by keeping the concentration of B constant. Let the order of reaction with respect to A be x. Then we will get the rate of the reaction as
Rate r = ${ k }_{ 1 }\left[ A \right] ^{ x }$
Let $\left[ { B }_{ 0 } \right]$ be the constant concentration of B.
${ k }_{ 1 } = { k }\left[ { B }_{ 0 } \right] ^{ y }$ where y is the order of reaction with respect to B.
Applying log to rate expression
\[\log { \left( r \right) } =\log { \left( k \right) + } x\log { \left( \left[ A \right] \right) }\]
We plot the above equation with log r on the y-axis and log [A] values on the x-axis. We find the slope of the straight line which is nothing but the order of reaction with respect to A.

Repeating the above procedure by keeping concentration A constant we get the order with respect to B. Combining these two orders we get the rate law of reaction. Adding orders of reaction with respect to each reactant we can get the total order of the reaction.
Note: This method is easy as we require less calculation to perform at a time. We can keep the reactant’s concentration common by taking that reactant concentration very large. As we take concentration large there will not be much change in the concentration of that reactant. So, it will nearly be constant.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




