
Explain Kolbe’s reaction with an equation.
Answer
593.7k+ views
Hint: Kolbe reaction is also called as Kolbe Schmitt reaction named after Herman and Rudolf Schmitt.
Kolbe reaction is a type of addition reaction. It is a chemical reaction which proceeds by heating sodium phenoxide with carbon dioxide under pressure, and then treating the product with sulphuric acid.
Complete answer:
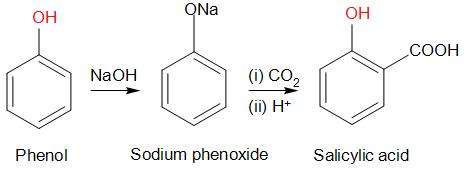
- Here we have taken phenol, and phenol is not directly reacted so, we have to remove the acidic hydrogen from it in presence of a base like NaOH. Which will change phenol to sodium phenoxide ion. We can see here that the phenoxide ion generated is more reactive than phenol towards the electrophilic aromatic substitution reaction.
- Then in the next step the sodium phenoxide ion is reacted with carbon dioxide and then followed by acidification, undergoes electrophilic substitution reaction to give ortho-hydroxybenzoic acid as the major product which is commonly called as salicylic acid.
- Hence, from this reaction of getting salicylic acid from phenol is called the Kolbe reaction.
Additional Information:
- There are various applications of Kolbe Reaction found like:
- It is also found that when Potassium Hydroxide is used in the Kolbe reaction, 4-Hydroxybenzoic acid can be accessed.
- We know that the salicylic acid can be used to make aspirin (which is a painkiller) by reacting it with acetic anhydride.
Note: We can see that Kolbe reaction is an addition reaction, which was mainly used to produce aromatic hydroxy acid. We can get the salicylic acid from phenol by the Kolbe reaction in presence of base like NaOH, and under pressure as well as in acidic conditions.
Kolbe reaction is a type of addition reaction. It is a chemical reaction which proceeds by heating sodium phenoxide with carbon dioxide under pressure, and then treating the product with sulphuric acid.
Complete answer:

- Here we have taken phenol, and phenol is not directly reacted so, we have to remove the acidic hydrogen from it in presence of a base like NaOH. Which will change phenol to sodium phenoxide ion. We can see here that the phenoxide ion generated is more reactive than phenol towards the electrophilic aromatic substitution reaction.
- Then in the next step the sodium phenoxide ion is reacted with carbon dioxide and then followed by acidification, undergoes electrophilic substitution reaction to give ortho-hydroxybenzoic acid as the major product which is commonly called as salicylic acid.
- Hence, from this reaction of getting salicylic acid from phenol is called the Kolbe reaction.
Additional Information:
- There are various applications of Kolbe Reaction found like:
- It is also found that when Potassium Hydroxide is used in the Kolbe reaction, 4-Hydroxybenzoic acid can be accessed.
- We know that the salicylic acid can be used to make aspirin (which is a painkiller) by reacting it with acetic anhydride.
Note: We can see that Kolbe reaction is an addition reaction, which was mainly used to produce aromatic hydroxy acid. We can get the salicylic acid from phenol by the Kolbe reaction in presence of base like NaOH, and under pressure as well as in acidic conditions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




