
Explain Liebermann's nitroso reaction with at least one example.
Answer
520.8k+ views
Hint: Liebermann's nitroso reaction can be used as a test to distinguish secondary ($2{}^\circ $) aliphatic or aromatic amines containing a nitroso group. Liebermann's reagent is composed of 10 ml of concentrated sulfuric acid (${{H}_{2}}S{{O}_{4}}$) and 1 g of potassium nitrate ($KN{{O}_{2}}$).
Complete answer:
The reaction between a phenol and concentrated sulfuric acid (${{H}_{2}}S{{O}_{4}}$) along with sodium nitrate ($NaN{{O}_{2}}$) given a product that has a deep blue or green color.
When this product obtained is diluted with water, it further turns into a red-colored solution.
This red-colored solution can be restored to its original deep blue or green color in the presence of alkaline sodium hydroxide (NaOH) or alkaline potassium hydroxide (KOH).
This process is known as Liebermann's nitroso reactions.
For example,
\[{{C}_{6}}{{H}_{5}}OH\xrightarrow[excess\text{ }{{H}_{2}}O]{NaN{{O}_{2}}}\operatorname{Re}d\xrightarrow[excess]{NaOH}Blue\]
Its mechanism is as follows
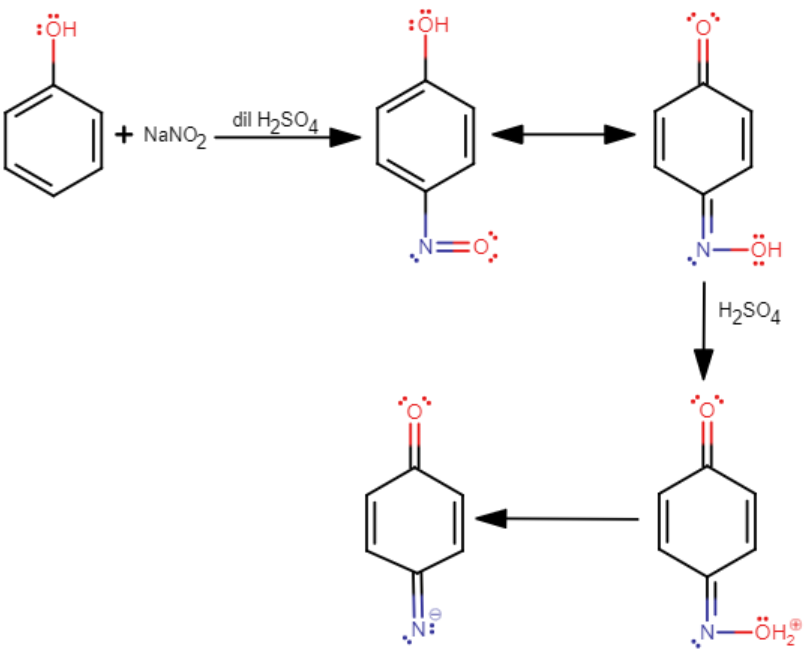
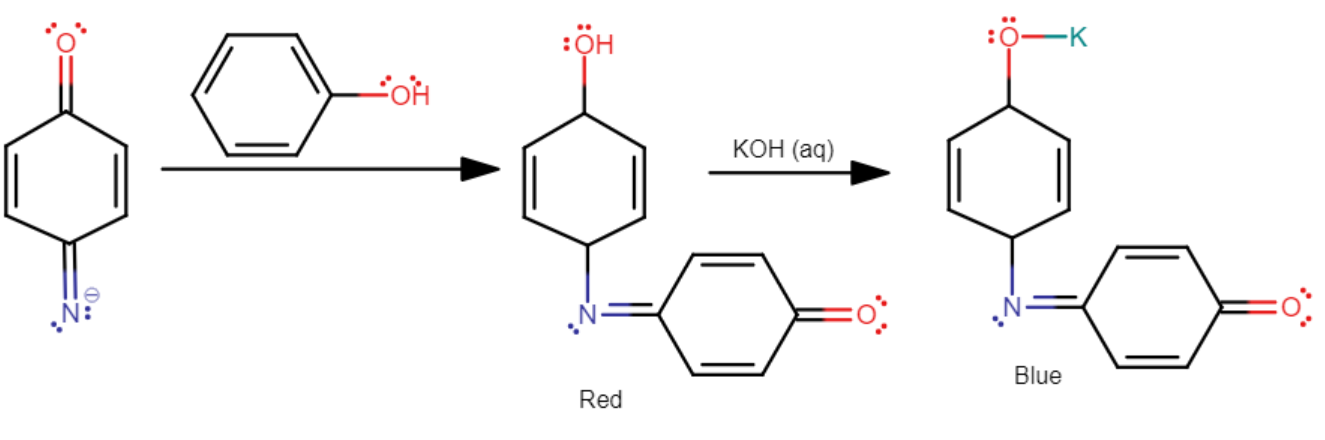
It should be noted that Liebermann's nitroso reaction is a test that is given only by secondary ($2{}^\circ $) aliphatic or aromatic amines. Upon treatment with nitric acid ($HN{{O}_{3}}$), the secondary ($2{}^\circ $) aliphatic or aromatic amine is converted into a nitroso amine. This nitroso amine, upon warming with phenol and concentrated sulfuric acid (${{H}_{2}}S{{O}_{4}}$) forms a red or brown color which first changes to blue, and then to green.
Upon dilution, the color changes to red, and upon further treatment with alkali, the color changes to violet or greenish-blue.
Note:
It should be noted that the test is performed by adding a drop of colorless and clear reagent to a small amount of substance that is scraped off. The analysis of the color of the resulting mixture obtained and the time taken for it to become apparent can help us determine the result.
Complete answer:
The reaction between a phenol and concentrated sulfuric acid (${{H}_{2}}S{{O}_{4}}$) along with sodium nitrate ($NaN{{O}_{2}}$) given a product that has a deep blue or green color.
When this product obtained is diluted with water, it further turns into a red-colored solution.
This red-colored solution can be restored to its original deep blue or green color in the presence of alkaline sodium hydroxide (NaOH) or alkaline potassium hydroxide (KOH).
This process is known as Liebermann's nitroso reactions.
For example,
\[{{C}_{6}}{{H}_{5}}OH\xrightarrow[excess\text{ }{{H}_{2}}O]{NaN{{O}_{2}}}\operatorname{Re}d\xrightarrow[excess]{NaOH}Blue\]
Its mechanism is as follows


It should be noted that Liebermann's nitroso reaction is a test that is given only by secondary ($2{}^\circ $) aliphatic or aromatic amines. Upon treatment with nitric acid ($HN{{O}_{3}}$), the secondary ($2{}^\circ $) aliphatic or aromatic amine is converted into a nitroso amine. This nitroso amine, upon warming with phenol and concentrated sulfuric acid (${{H}_{2}}S{{O}_{4}}$) forms a red or brown color which first changes to blue, and then to green.
Upon dilution, the color changes to red, and upon further treatment with alkali, the color changes to violet or greenish-blue.
Note:
It should be noted that the test is performed by adding a drop of colorless and clear reagent to a small amount of substance that is scraped off. The analysis of the color of the resulting mixture obtained and the time taken for it to become apparent can help us determine the result.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




