
Explain R.S. nomenclature with illustrations.
Answer
565.2k+ views
Hint:The R and S nomenclature is helpful for the identification of the arrangements of groups on the enantiomers around the chiral centre.
R refers to rectus which means right in Latin and S refers to Sinister which means left in Latin.
Complete answer:
We know that for an optically active molecule there will be a chiral centre or chiral carbon.
Chiral carbon is the carbon atom in which the four groups attached to them will be entirely different groups.
R and S configuration is very much helpful for us to identify the enantiomers.
In R and S nomenclature, we assign a number, according to the CIP rules for each group of atoms or atoms that is attached with the chiral carbon.
CIP rules means Cahn-Ingold-Prelog prioritizing rules and the substituents present in the molecule is assigned according to this rule. According to CIP rule, the substituent with the highest atomic number will be assigned as 1 and next as 2 and then 3 and the last one will be 4.
So in short we can say that, the CIP rule for prioritizing substituents is according to the atomic number of the atom.
And we will find whether the enantiomer is having R is S configuration, in such a way that, after assigning the groups if the arrangement of the substituents is in clockwise direction then the molecule possess the R configuration and if the substituents are arranged in anti-clockwise fashion then the molecule is having the S configuration.
For more clarity let’s consider an example,
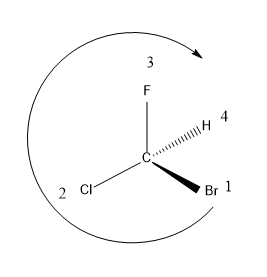
In this structure, Br is the substituent with highest atomic number hence it is assigned as the first group then Cl, F and H.
H is the lowest priority group (LGP) and the bond between H and the chiral C is represented in dash representation i.e. the bond is away from the observer. The LGP should always be present away from the observer.
After assigning the groups, let’s trace the direction of arrangement of the substituents from the highest priority group to lowest priority group.
As we have traced the direction in the figure, the arrangement of substituents is in the clockwise direction. Hence we could say that the molecule is having R configuration.
Now let’s see if the structure was having S configuration, then what will be the arrangement of the substituents.
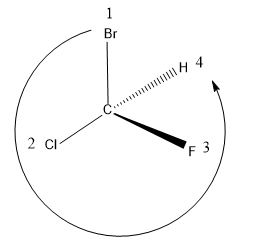
Here the same molecule is represented, but here the substituents are arranged in space in different fashion.
After assigning the groups, if we trace the direction of the substituents, the direction of HGP to LGP is in the anti-clockwise direction i.e. in S configuration.
Note:
If the lowest priority group is not present in the dash bond position of the molecule i.e. in the position away from the observer, then we have to carry out rotation so that the groups are interchanged and the LGP gets the dashed position. Then only the numbers should be assigned and the configuration should be determined.
R refers to rectus which means right in Latin and S refers to Sinister which means left in Latin.
Complete answer:
We know that for an optically active molecule there will be a chiral centre or chiral carbon.
Chiral carbon is the carbon atom in which the four groups attached to them will be entirely different groups.
R and S configuration is very much helpful for us to identify the enantiomers.
In R and S nomenclature, we assign a number, according to the CIP rules for each group of atoms or atoms that is attached with the chiral carbon.
CIP rules means Cahn-Ingold-Prelog prioritizing rules and the substituents present in the molecule is assigned according to this rule. According to CIP rule, the substituent with the highest atomic number will be assigned as 1 and next as 2 and then 3 and the last one will be 4.
So in short we can say that, the CIP rule for prioritizing substituents is according to the atomic number of the atom.
And we will find whether the enantiomer is having R is S configuration, in such a way that, after assigning the groups if the arrangement of the substituents is in clockwise direction then the molecule possess the R configuration and if the substituents are arranged in anti-clockwise fashion then the molecule is having the S configuration.
For more clarity let’s consider an example,

In this structure, Br is the substituent with highest atomic number hence it is assigned as the first group then Cl, F and H.
H is the lowest priority group (LGP) and the bond between H and the chiral C is represented in dash representation i.e. the bond is away from the observer. The LGP should always be present away from the observer.
After assigning the groups, let’s trace the direction of arrangement of the substituents from the highest priority group to lowest priority group.
As we have traced the direction in the figure, the arrangement of substituents is in the clockwise direction. Hence we could say that the molecule is having R configuration.
Now let’s see if the structure was having S configuration, then what will be the arrangement of the substituents.

Here the same molecule is represented, but here the substituents are arranged in space in different fashion.
After assigning the groups, if we trace the direction of the substituents, the direction of HGP to LGP is in the anti-clockwise direction i.e. in S configuration.
Note:
If the lowest priority group is not present in the dash bond position of the molecule i.e. in the position away from the observer, then we have to carry out rotation so that the groups are interchanged and the LGP gets the dashed position. Then only the numbers should be assigned and the configuration should be determined.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




