
Explain the chemiosmotic hypothesis for ATP synthesis.
Answer
583.2k+ views
Hint: A chemiosmotic Hypothesis may be an organic process that was theorized in 1961 by a British biochemist Peter Dennis Mitchell postulated this hypothesis which explains the mechanism of synthesis of ATP during photosynthesis, within the chloroplast.
Complete answer:
This hypothesis stated that a proton-motive force was liable for driving the synthesis of ATP. During this hypothesis, protons would be pumped across the inner mitochondrial membrane as electrons went through the electron transfer chain. This is able to end in a proton gradient with a lower pH within the intermembrane space and an elevated pH within the matrix of the mitochondria. An intact inner mitochondrial membrane, impermeable to protons, may be a requirement of such a model. The proton gradient and membrane potential are the proton-motive force that drive ATP synthesis. These electron carriers are sites of redox reaction for electrons and with each reaction across the electron carriers the energy of the electron is transferred into the pumping of hydrogen ions across the membrane, this leads to a higher concentration within the intermembrane space than that of the matrix. The protons undergo the ATP synthase from a neighborhood of high concentration within the intermembrane space to a neighborhood of lower concentration, the mitochondrial matrix, through facilitated diffusion generating ATP. Mitchell's chemiosmotic hypothesis has been widely accepted because of the mechanism of the coupling of electron transport and ATP synthesis. He was awarded the Nobel prize in Chemistry in 1978.
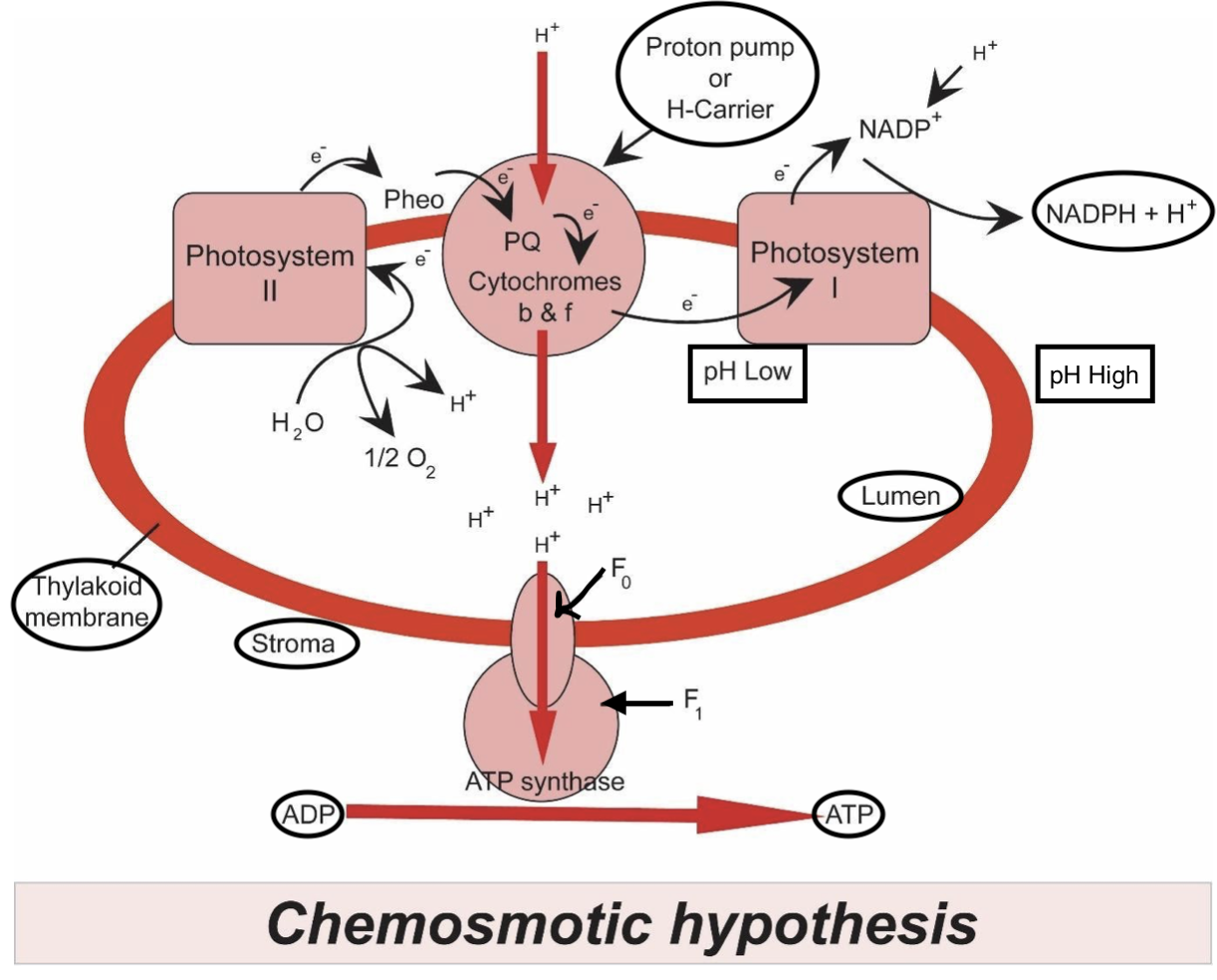
Plants during the light-dependent reaction gain energy from sunlight in photosystems as a way of getting electrons from a lower energy state to a better energy state. The supply of this electron is from the photolysis of water, which additionally generates protons wanted for chemiosmosis FAD and NAD coenzymes are the source in mitochondria springs. During the previous steps of respiration, especially the Krebs cycle, the reduction of coenzymes plays a pivotal role as a supply of electrons and protons for the electron transport chain and producing the proton-motive force.
Note: The proton-motive force is that the mathematical sum of the chemical gradient expressed because the difference in pH between the matrix and intermembrane space, and therefore the charge gradient created by the disequilibrium (via proton pumping) of proton distribution either side of the inner membrane In effect, the pH gradient acts as a "battery" which stores energy to supply ATP. In both plants and animals, oxygen is employed because the commonest final electron acceptor in order that the electron transport chain can continue in order that chemiosmosis and production of ATP can continue. Inhibitors like cyanide can block the aforementioned process leading to no ATP production and subsequently death.
Complete answer:
This hypothesis stated that a proton-motive force was liable for driving the synthesis of ATP. During this hypothesis, protons would be pumped across the inner mitochondrial membrane as electrons went through the electron transfer chain. This is able to end in a proton gradient with a lower pH within the intermembrane space and an elevated pH within the matrix of the mitochondria. An intact inner mitochondrial membrane, impermeable to protons, may be a requirement of such a model. The proton gradient and membrane potential are the proton-motive force that drive ATP synthesis. These electron carriers are sites of redox reaction for electrons and with each reaction across the electron carriers the energy of the electron is transferred into the pumping of hydrogen ions across the membrane, this leads to a higher concentration within the intermembrane space than that of the matrix. The protons undergo the ATP synthase from a neighborhood of high concentration within the intermembrane space to a neighborhood of lower concentration, the mitochondrial matrix, through facilitated diffusion generating ATP. Mitchell's chemiosmotic hypothesis has been widely accepted because of the mechanism of the coupling of electron transport and ATP synthesis. He was awarded the Nobel prize in Chemistry in 1978.

Plants during the light-dependent reaction gain energy from sunlight in photosystems as a way of getting electrons from a lower energy state to a better energy state. The supply of this electron is from the photolysis of water, which additionally generates protons wanted for chemiosmosis FAD and NAD coenzymes are the source in mitochondria springs. During the previous steps of respiration, especially the Krebs cycle, the reduction of coenzymes plays a pivotal role as a supply of electrons and protons for the electron transport chain and producing the proton-motive force.
Note: The proton-motive force is that the mathematical sum of the chemical gradient expressed because the difference in pH between the matrix and intermembrane space, and therefore the charge gradient created by the disequilibrium (via proton pumping) of proton distribution either side of the inner membrane In effect, the pH gradient acts as a "battery" which stores energy to supply ATP. In both plants and animals, oxygen is employed because the commonest final electron acceptor in order that the electron transport chain can continue in order that chemiosmosis and production of ATP can continue. Inhibitors like cyanide can block the aforementioned process leading to no ATP production and subsequently death.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




