
Explain the following reaction:
(a)Sandmeyer reaction
(b)Wurtz’s Fittig reaction
Answer
567k+ views
Hint: The reactions mentioned are basically named reactions, these reactions are named after their discoverer or developer. Many reactions have scientific names but these on contrary are named after some person. The above-mentioned reactions are organic chemical reactions in which halides have a significant role.
Complete answer:
Sandmeyer reaction
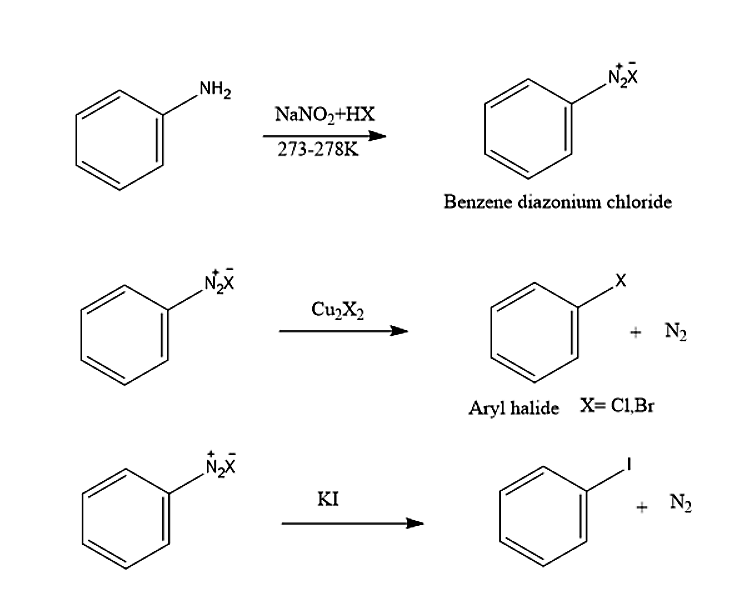
The Sandmeyer reaction is a chemical reaction used for synthesis of aryl halides from aryl diazonium salts using copper salts as catalysts or reagent. It is an example of a radical-nucleophilic aromatic substitution. This reaction is named after Traugott Sandmeyer.
When a primary aromatic amine is dissolved or suspended in cold aqueous mineral acid, is treated with sodium nitrite, a diazonium salt is formed. Mixing the solution of freshly prepared diazonium salt with cuprous chloride or cuprous bromide leads in the replacement of the diazonium group by -Cl or -Br.
Wurtz’s Fittig reaction:
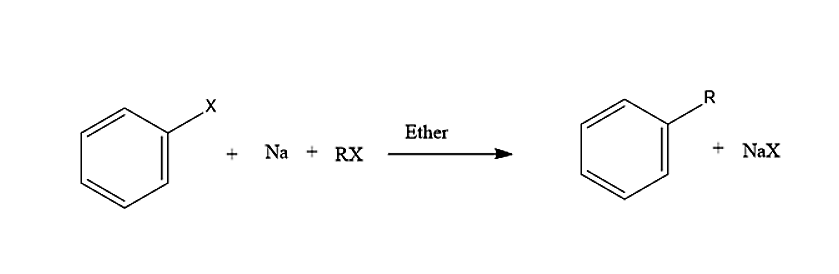
A mixture of an haloalkane and aryl halide gives an alkyl arene when treated with sodium in dry ether and is called Wurtz-Fittig reaction. This reaction is named after Charles Adolphe Wurtz and Wilhelm Rudolph Fittig.
There are two approaches to describing the mechanism of the Wurtz-Fittig reaction.
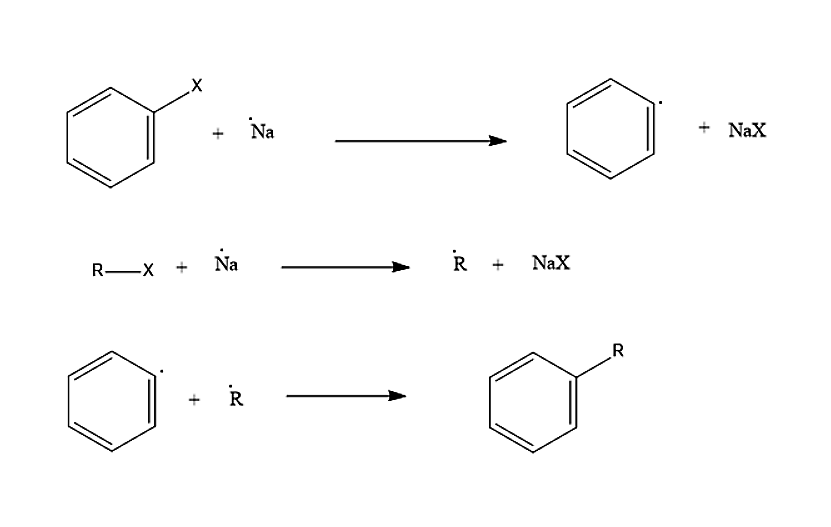
The first involves sodium mediated formation of both alkyl and aryl radicals then combine to form a substituted aromatic compound. In simple words we can say Radical Mechanism for the Wurtz-Fittig reaction.
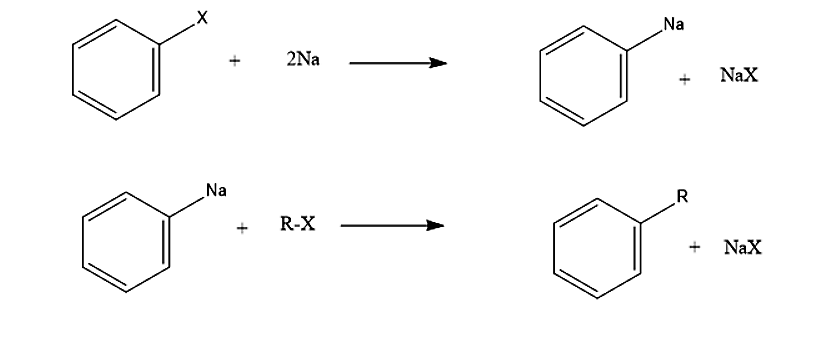
The second approach involves the formation of an intermediate organometallic compound followed by nucleophilic attack of the alkyl halide.
Note:
The reaction mechanism is based on Substitution Reaction.
The Sandmeyer reaction provide us a method through which one can perform unique transformations on benzene, such as halogenation, cyanation, trifluoromethylation and hydroxylation
Replacement of the diazonium group by iodine does not require the presence of cuprous halide and is done simply by shaking the diazonium salt with potassium iodide.
The type Wurtz’s Fittig reaction of reaction is Coupling reaction.
The Wurtz’s Fittig reaction works best for the asymmetrical products if the halide reactants are somehow separate in their chemical properties.
Complete answer:
Sandmeyer reaction
The Sandmeyer reaction is a chemical reaction used for synthesis of aryl halides from aryl diazonium salts using copper salts as catalysts or reagent. It is an example of a radical-nucleophilic aromatic substitution. This reaction is named after Traugott Sandmeyer.
When a primary aromatic amine is dissolved or suspended in cold aqueous mineral acid, is treated with sodium nitrite, a diazonium salt is formed. Mixing the solution of freshly prepared diazonium salt with cuprous chloride or cuprous bromide leads in the replacement of the diazonium group by -Cl or -Br.

Wurtz’s Fittig reaction:

A mixture of an haloalkane and aryl halide gives an alkyl arene when treated with sodium in dry ether and is called Wurtz-Fittig reaction. This reaction is named after Charles Adolphe Wurtz and Wilhelm Rudolph Fittig.
There are two approaches to describing the mechanism of the Wurtz-Fittig reaction.
The first involves sodium mediated formation of both alkyl and aryl radicals then combine to form a substituted aromatic compound. In simple words we can say Radical Mechanism for the Wurtz-Fittig reaction.

The second approach involves the formation of an intermediate organometallic compound followed by nucleophilic attack of the alkyl halide.

Note:
The reaction mechanism is based on Substitution Reaction.
The Sandmeyer reaction provide us a method through which one can perform unique transformations on benzene, such as halogenation, cyanation, trifluoromethylation and hydroxylation
Replacement of the diazonium group by iodine does not require the presence of cuprous halide and is done simply by shaking the diazonium salt with potassium iodide.
The type Wurtz’s Fittig reaction of reaction is Coupling reaction.
The Wurtz’s Fittig reaction works best for the asymmetrical products if the halide reactants are somehow separate in their chemical properties.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




