
Explain the following terms:
(a). Homopolymer
(b). Elastomers
Answer
583.5k+ views
Hint: We know that the polymer is that giant unit which can be manufactured by several small units. The process of making polymers from small units is termed as polymerization reaction. They are usually like plastic.
(a). Homopolymer:
The homopolymer is that kind of polymer that is made up of the same monomer unit. The word homo means similar. As we know, the polymer polythene is made up of one type of monomer that is ethene.
The several units of ethane are combined together to form polymer polythene. The reaction from which polythene is made, as shown below.
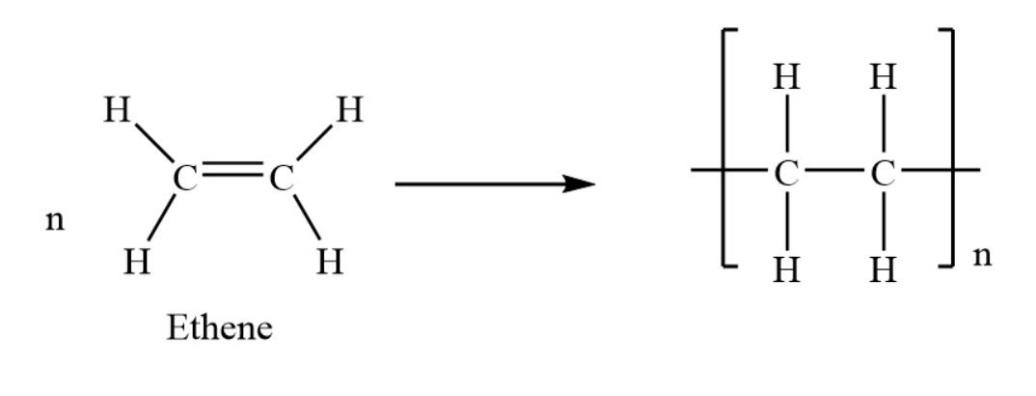
The polymer of polythene is cheap and flexible. The other examples of homopolymer include nylon −6, polystyrene, etc.
(b).Elastomer:
The elastomers are those types of polymer which show the property named as viscoelasticity. The general meaning of viscoelasticity is the combination of viscosity and elasticity property. The elasticity is the property in which the substance regains its natural form after searching. They have a very weak intermolecular force between the chemical species present in the elastomer. Some of the examples of elastomers are neoprene, vulcanized rubber. The structure of the elastomer is shown below.
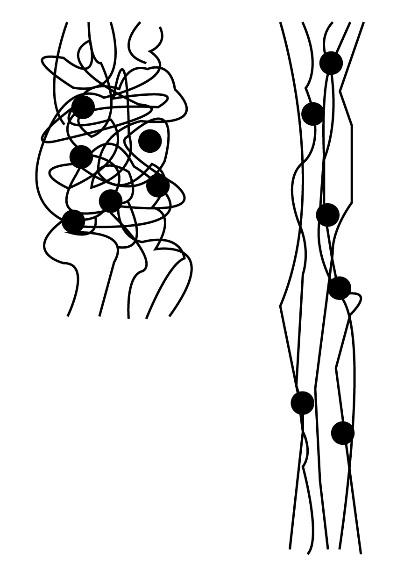
Note:
The uses of the polymer are discussed below.
- The polymer polythene is used in the packaging.
- The polymer poly chloroethene can be used in making electricity wires, pipes and windows.
- The polymer polytetrafluroethene can be used to make laboratory substances and also used for making non-stick pans.
- The polymer polypropylene can be used to make ropes, buckets, etc.
(a). Homopolymer:
The homopolymer is that kind of polymer that is made up of the same monomer unit. The word homo means similar. As we know, the polymer polythene is made up of one type of monomer that is ethene.
The several units of ethane are combined together to form polymer polythene. The reaction from which polythene is made, as shown below.

The polymer of polythene is cheap and flexible. The other examples of homopolymer include nylon −6, polystyrene, etc.
(b).Elastomer:
The elastomers are those types of polymer which show the property named as viscoelasticity. The general meaning of viscoelasticity is the combination of viscosity and elasticity property. The elasticity is the property in which the substance regains its natural form after searching. They have a very weak intermolecular force between the chemical species present in the elastomer. Some of the examples of elastomers are neoprene, vulcanized rubber. The structure of the elastomer is shown below.

Note:
The uses of the polymer are discussed below.
- The polymer polythene is used in the packaging.
- The polymer poly chloroethene can be used in making electricity wires, pipes and windows.
- The polymer polytetrafluroethene can be used to make laboratory substances and also used for making non-stick pans.
- The polymer polypropylene can be used to make ropes, buckets, etc.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




