
Explain the following with one example.
(A) Aldol condensation
(B) Cannizzaro reaction
(C) Esterification
(D) Decarboxylation
Answer
530.7k+ views
Hint: Aldol condensation occurs between two aldehydes or a ketone molecule to form $ \beta - $ hydroxy aldehyde or ketone.
In Cannizzaro reaction two aldehyde molecules react to give alcohol and carboxylic acid.
The process of obtaining esters by the reaction of alcohol and carboxylic acid is known as esterification.
Decarboxylation reaction as the name suggests is the removal of $ C{O_2} $ by eliminating a carboxyl group.
Complete step by step answer:
In aldol condensation, the reaction of an enolate ion with a carbonyl compound in the presence of a dilute base to give $ \beta - $ hydroxy aldehyde or ketone. The reaction takes place only if the aldehyde has an $ \alpha - H $ .
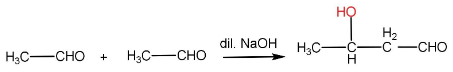
Step-I: Formation of enolate ion from $ {1^{st}} $ aldehyde molecule
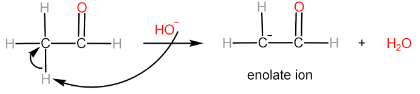
Step-II: Attack of enolate ion on $ {2^{nd}} $ aldehyde molecule
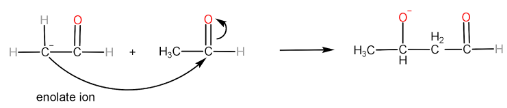
Step-III: Formation of aldol and regeneration of $ O{H^ - } $ ion
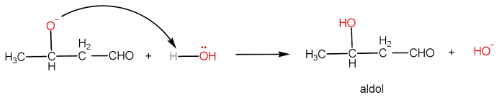
Cannizzaro reaction is the formation of a primary alcohol and a carboxylic acid molecule by the reaction of two aldehyde molecules in the presence of a strong base. The participating aldehyde molecules should not have any $ \alpha - H $ , as such molecules readily form enolate ions and the reaction does not occur.

Step-I: Attack of $ O{H^ - } $ ion on $ {1^{st}} $ aldehyde molecule
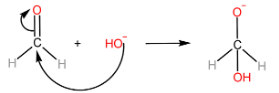
Step-II: Transfer of hydride ion to the $ {2^{nd}} $ aldehyde molecule, followed by protonation of alkoxide ion to form alcohol.
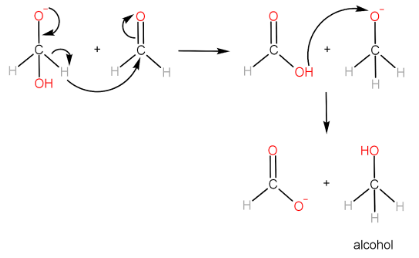
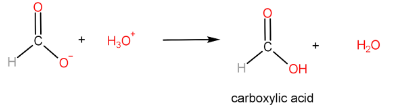
Step-III: Protonation of carboxylate ion to form carboxylic acid
The reaction of carboxylic acid with a primary alcohol in the presence of sulphuric acid to form an ester is called esterification reaction.
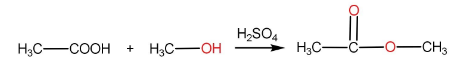
Step-I: Protonation of carboxylic acid to form carbocation
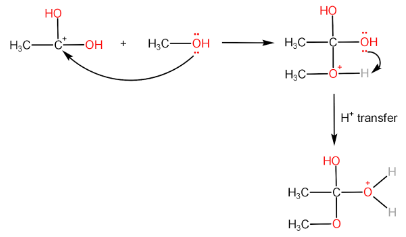
Step-II: Attack of alcohol on the carbocation followed by proton transfer within the molecule.
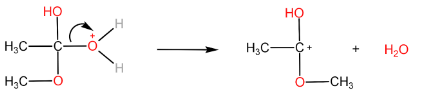
Step-III: Removal of $ {H_2}O $ molecule.
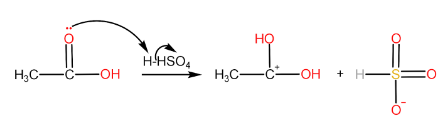
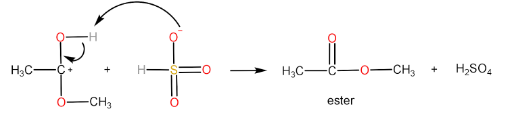
Step-IV: Removal of $ {H^ + } $ ion by $ HSO_4^ - $ to form ester and $ {H_2}S{O_4} $
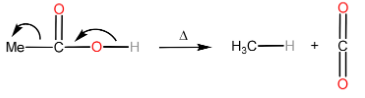
Decarboxylation is the removal of a carboxyl group thereby releasing $ C{O_2} $ usually from a carboxylic acid to form a product with one carbon less than the reacting carboxylic acid.
Note:
Mostly there is a confusion between the aldol condensation and the Cannizzaro reaction. We have to remember that in aldol condensation two aldehyde molecules having $ \alpha - H $ react with each other in the presence of dilute base while in Cannizzaro reaction two aldehyde molecules which do not have $ \alpha - H $ react in the presence of strong base.
In Cannizzaro reaction two aldehyde molecules react to give alcohol and carboxylic acid.
The process of obtaining esters by the reaction of alcohol and carboxylic acid is known as esterification.
Decarboxylation reaction as the name suggests is the removal of $ C{O_2} $ by eliminating a carboxyl group.
Complete step by step answer:
In aldol condensation, the reaction of an enolate ion with a carbonyl compound in the presence of a dilute base to give $ \beta - $ hydroxy aldehyde or ketone. The reaction takes place only if the aldehyde has an $ \alpha - H $ .

Step-I: Formation of enolate ion from $ {1^{st}} $ aldehyde molecule

Step-II: Attack of enolate ion on $ {2^{nd}} $ aldehyde molecule

Step-III: Formation of aldol and regeneration of $ O{H^ - } $ ion

Cannizzaro reaction is the formation of a primary alcohol and a carboxylic acid molecule by the reaction of two aldehyde molecules in the presence of a strong base. The participating aldehyde molecules should not have any $ \alpha - H $ , as such molecules readily form enolate ions and the reaction does not occur.

Step-I: Attack of $ O{H^ - } $ ion on $ {1^{st}} $ aldehyde molecule

Step-II: Transfer of hydride ion to the $ {2^{nd}} $ aldehyde molecule, followed by protonation of alkoxide ion to form alcohol.

Step-III: Protonation of carboxylate ion to form carboxylic acid

The reaction of carboxylic acid with a primary alcohol in the presence of sulphuric acid to form an ester is called esterification reaction.

Step-I: Protonation of carboxylic acid to form carbocation

Step-II: Attack of alcohol on the carbocation followed by proton transfer within the molecule.

Step-III: Removal of $ {H_2}O $ molecule.

Step-IV: Removal of $ {H^ + } $ ion by $ HSO_4^ - $ to form ester and $ {H_2}S{O_4} $

Decarboxylation is the removal of a carboxyl group thereby releasing $ C{O_2} $ usually from a carboxylic acid to form a product with one carbon less than the reacting carboxylic acid.

Note:
Mostly there is a confusion between the aldol condensation and the Cannizzaro reaction. We have to remember that in aldol condensation two aldehyde molecules having $ \alpha - H $ react with each other in the presence of dilute base while in Cannizzaro reaction two aldehyde molecules which do not have $ \alpha - H $ react in the presence of strong base.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




