
Explain the formation of a chemical bond.
Answer
592.5k+ views
Hint: A chemical bond is formed as a result of forces of attraction due to electrostatic forces. This brings the reacting species together and helps their orbital with equal energies and proper orientation to overlap and form molecular orbitals.
Complete answer:
A chemical bond is a formation of bond due to the electrostatic forces of attraction due to sharing of electrons or donating electrons. The attractive force which holds up the constituent particles (atoms, ions or molecules) together in a chemical species is known as chemical bond.
Atoms either share or gain or lose electrons to attain stable electronic configuration. Due to this, a state of minimum energy is obtained and a chemical bond is formed. This results in maximum stability. When two atoms share electrons, a covalent bond is formed and when atoms lose or gain electrons, ionic bond is formed.
Chemical bond are of three type which are as follows:
Ionic bonds, covalent bonds and metallic bonds.
Ionic bonds:
Ionic bond is formed when a metal cation is bonded to anion of a non-metal that is metal donates electron to anion and in this case both cation and anion complete their octet.
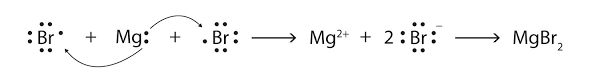
Covalent bond:
A covalent bond occurs when electrons are shared between the two or more bonding atoms to complete - an octet.
Example given:

Electrons are shared as per the requirement of each atom involved.
Note: There are exceptions to octet rule. For example in the case of Hydrogen and helium a duet configuration is stable.
Complete answer:
A chemical bond is a formation of bond due to the electrostatic forces of attraction due to sharing of electrons or donating electrons. The attractive force which holds up the constituent particles (atoms, ions or molecules) together in a chemical species is known as chemical bond.
Atoms either share or gain or lose electrons to attain stable electronic configuration. Due to this, a state of minimum energy is obtained and a chemical bond is formed. This results in maximum stability. When two atoms share electrons, a covalent bond is formed and when atoms lose or gain electrons, ionic bond is formed.
Chemical bond are of three type which are as follows:
Ionic bonds, covalent bonds and metallic bonds.
Ionic bonds:
Ionic bond is formed when a metal cation is bonded to anion of a non-metal that is metal donates electron to anion and in this case both cation and anion complete their octet.
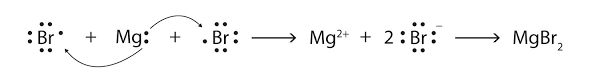
Covalent bond:
A covalent bond occurs when electrons are shared between the two or more bonding atoms to complete - an octet.
Example given:

Electrons are shared as per the requirement of each atom involved.
Note: There are exceptions to octet rule. For example in the case of Hydrogen and helium a duet configuration is stable.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




