
Explain the formation of a chemical bond.
Answer
581.7k+ views
Hint: Basically, a chemical bond is a lasting attraction between atoms, ions or molecules that enable the formation of chemical compounds. Moreover, the bond may result from the electrostatic force of attraction between oppositely charged ions as in case of ionic bonds or through the sharing of electrons as in covalent bonds.
Complete step by step answer:
Basically, the chemical bonding refers to the formation of a chemical bond between two or more atoms, molecules or ions to give rise to a chemical compound. Further, the bond formation is accompanied by lowering the energy of the system. Now, in case of atoms where the electron transfer takes place, a chemical bond may be formed in such a manner which may lead to lowering the energy of the system.
Now, the attractive forces which hold together the constituent particles such as atoms, ions or molecules in a chemical species is known as chemical bond. The atoms either share or gain or lose electrons to attain stable electronic configuration. So, due to this, a state of minimum energy is obtained and a chemical bond is formed. Moreover, when two atoms share electrons, then a covalent bond is formed and when atoms lose or gain electrons then ionic bond is formed. Now, you can see the bond formation in the picture given below:
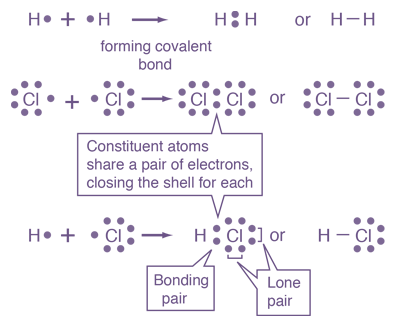
Note: Other types of chemical bonds are hydrogen bonds and polar bonds. Hydrogen bonding is a weaker form of chemical bonding. This type of bonding occurs between oxygen and hydrogen wherein the hydrogen develops a partial positive charge. Moreover, in polar covalent bonding, the electrons are shared unequally because the more electronegative atom pulls the electron pair closer to itself and away from the less electronegative atom.
Complete step by step answer:
Basically, the chemical bonding refers to the formation of a chemical bond between two or more atoms, molecules or ions to give rise to a chemical compound. Further, the bond formation is accompanied by lowering the energy of the system. Now, in case of atoms where the electron transfer takes place, a chemical bond may be formed in such a manner which may lead to lowering the energy of the system.
Now, the attractive forces which hold together the constituent particles such as atoms, ions or molecules in a chemical species is known as chemical bond. The atoms either share or gain or lose electrons to attain stable electronic configuration. So, due to this, a state of minimum energy is obtained and a chemical bond is formed. Moreover, when two atoms share electrons, then a covalent bond is formed and when atoms lose or gain electrons then ionic bond is formed. Now, you can see the bond formation in the picture given below:

Note: Other types of chemical bonds are hydrogen bonds and polar bonds. Hydrogen bonding is a weaker form of chemical bonding. This type of bonding occurs between oxygen and hydrogen wherein the hydrogen develops a partial positive charge. Moreover, in polar covalent bonding, the electrons are shared unequally because the more electronegative atom pulls the electron pair closer to itself and away from the less electronegative atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




