
Explain the formation of a double bond in an oxygen molecule.
Answer
569.4k+ views
Hint: We need to know the concept of chemical bonding, covalent bonds and the octet rule. The attractive force which holds various constituents such as atoms and ions together in different chemical species is called a chemical bond. A covalent bond is a chemical bond that is formed by the sharing of electron pairs between atoms.
Complete step by step answer:
We have to remember that a strict rule is followed by atoms during covalent bonding which is the octet rule. According to this rule, main group elements tend to bond in such a way, either by losing, gaining or sharing electrons such that each atom has 8 electrons in its outermost (valence shell).
To further understand the covalent bond concept between molecules, a theory comes into picture. This theory is the Lewis-Langmuir theory where electrons are represented as dots and the bonding between atoms can be shown with the help of structures referred to as Lewis dot structures. The following conditions must be followed to write the Lewis dot structure of molecules:
Each bond is formed as a result of sharing an electron pair between the atoms.
Each combining atom contributes at least one electron to the shared pair.
The combining atoms attain the outer-shell noble gas configuration as a result of sharing of electrons.
Thus, when two atoms share one electron pair, they are said to be bonded by a single covalent bond. For example \[C{l_{2\;}}\] molecule. \[Cl\] Has \[7\] electrons in its valence shell with the electronic configuration \[\left[ {Ne} \right]3{s^2}3{p^5}\].To complete its octet, it requires one electron.
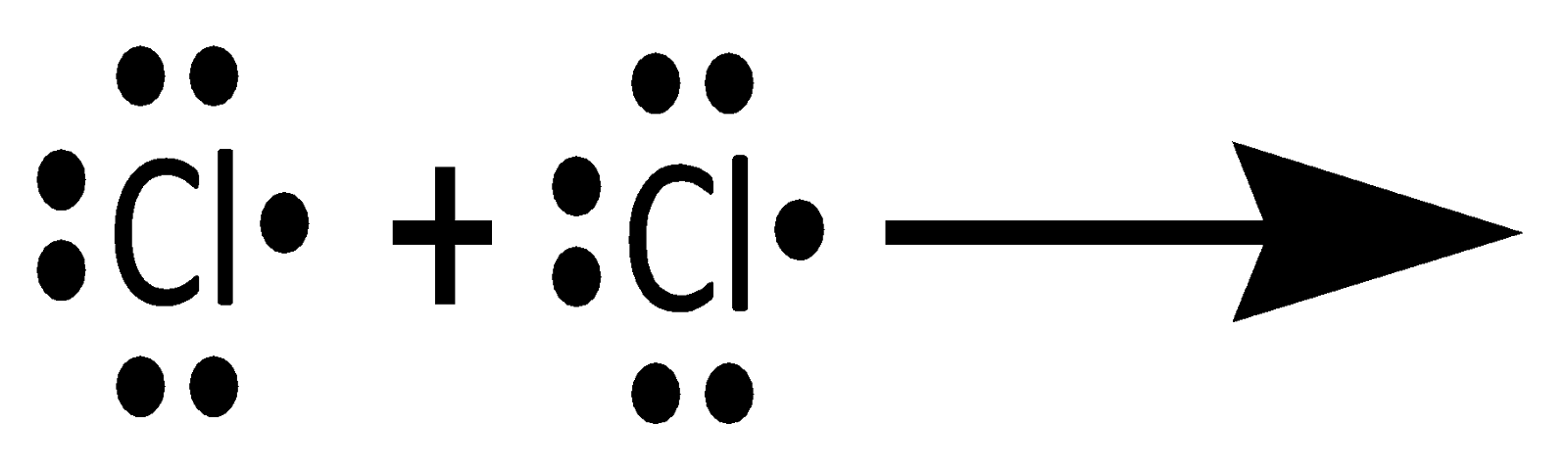

Thus, the formation of the \[C{l_{2\;}}\] can be understood by sharing a pair of electrons, i.e. a single covalent bond. In this process, both the chlorine atoms attain the outer shell octet of the nearest noble gas i.e. Argon.
In the similar way, many compounds have multiple bonds between atoms by sharing electrons. The double bond in an oxygen molecule can be explained by understanding the covalent bonding between the oxygen atoms. \[O\] has the electronic configuration \[\left[ {He} \right]2{s^2}2{p^4}\]. To complete its octet, it requires two electrons. Hence the Lewis-dot structure can be written as follows:
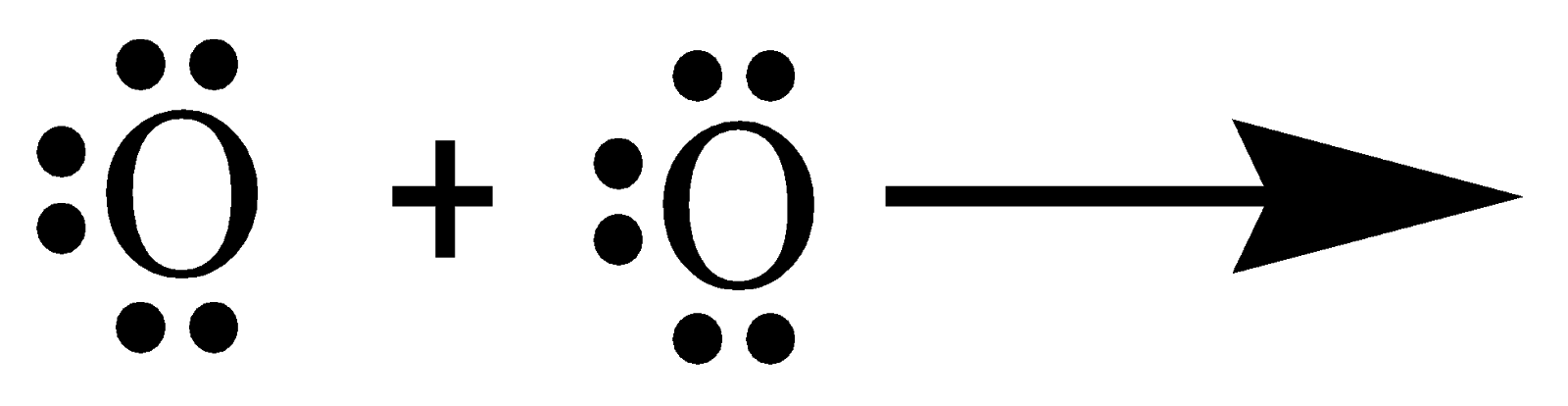
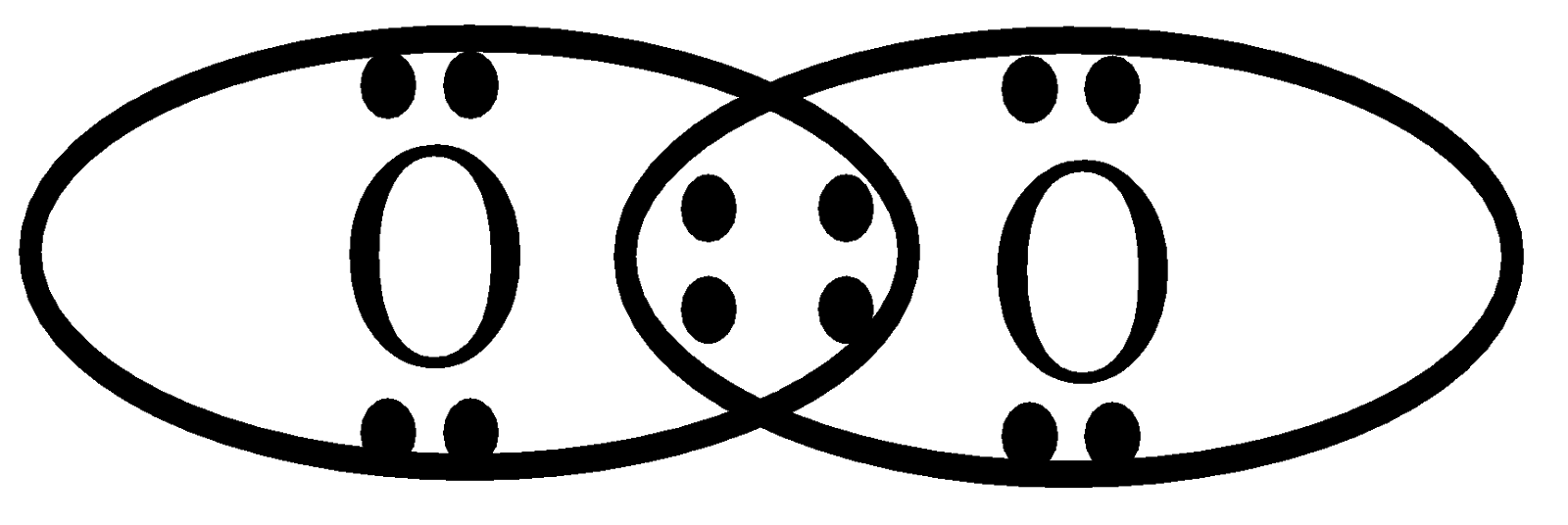
Thus, the formation of an oxygen molecule (\[{O_2}\]) is by the sharing of two pairs of electrons, i.e. a double covalent bond.
Note: It must be noted that though the octet rule is useful, it is not universal as it has some exceptions or drawbacks and limitations. In some compounds such as \[LiCl\], \[Be{H_2}\], \[AlC{l_3}\], etc., the number of electrons surrounding the central atom (whose valence electrons are less than four) is less than eight after the bonding. Also, in molecules with an odd number of electrons like \[NO\] and \[N{O_2}\], the octet rule is not satisfied for all the atoms. Furthermore, some compounds such as PF5, there are more than eight valence electrons around the central atom (expanded octet).Other drawbacks of the octet rule are:
(i) This theory is not helpful for the prediction of shape of molecules.
(ii) Although octet rule is based on the chemical inertness of noble gases, some noble gases do combine with oxygen and fluorine to form compounds such as $XeOF_3$.
(iii) This rule does not explain the relative stability of molecules.
Complete step by step answer:
We have to remember that a strict rule is followed by atoms during covalent bonding which is the octet rule. According to this rule, main group elements tend to bond in such a way, either by losing, gaining or sharing electrons such that each atom has 8 electrons in its outermost (valence shell).
To further understand the covalent bond concept between molecules, a theory comes into picture. This theory is the Lewis-Langmuir theory where electrons are represented as dots and the bonding between atoms can be shown with the help of structures referred to as Lewis dot structures. The following conditions must be followed to write the Lewis dot structure of molecules:
Each bond is formed as a result of sharing an electron pair between the atoms.
Each combining atom contributes at least one electron to the shared pair.
The combining atoms attain the outer-shell noble gas configuration as a result of sharing of electrons.
Thus, when two atoms share one electron pair, they are said to be bonded by a single covalent bond. For example \[C{l_{2\;}}\] molecule. \[Cl\] Has \[7\] electrons in its valence shell with the electronic configuration \[\left[ {Ne} \right]3{s^2}3{p^5}\].To complete its octet, it requires one electron.
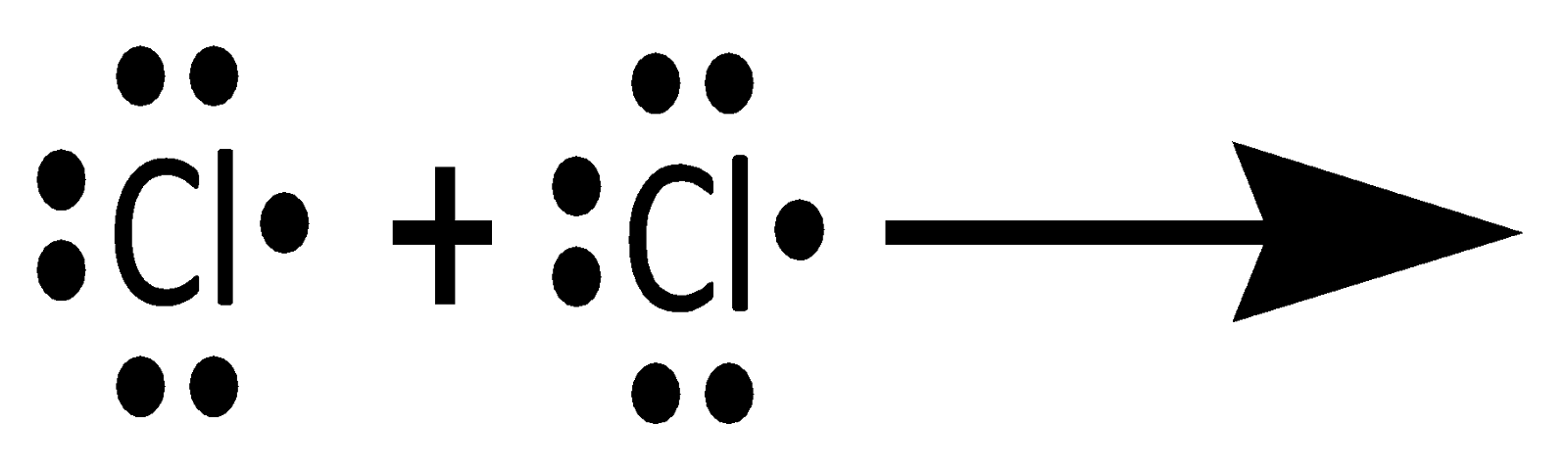

Thus, the formation of the \[C{l_{2\;}}\] can be understood by sharing a pair of electrons, i.e. a single covalent bond. In this process, both the chlorine atoms attain the outer shell octet of the nearest noble gas i.e. Argon.
In the similar way, many compounds have multiple bonds between atoms by sharing electrons. The double bond in an oxygen molecule can be explained by understanding the covalent bonding between the oxygen atoms. \[O\] has the electronic configuration \[\left[ {He} \right]2{s^2}2{p^4}\]. To complete its octet, it requires two electrons. Hence the Lewis-dot structure can be written as follows:
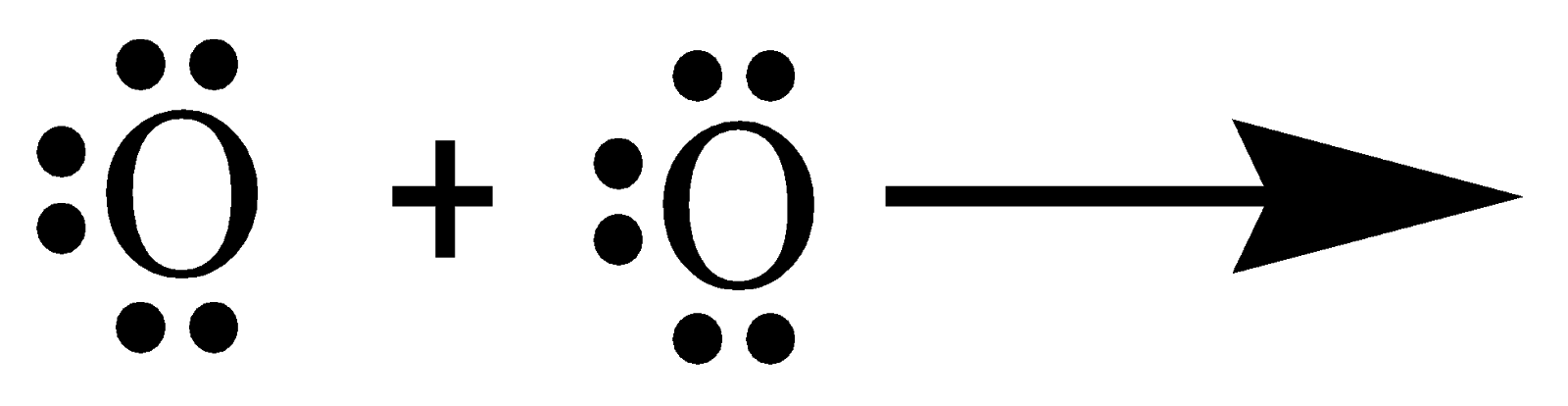

Thus, the formation of an oxygen molecule (\[{O_2}\]) is by the sharing of two pairs of electrons, i.e. a double covalent bond.
Note: It must be noted that though the octet rule is useful, it is not universal as it has some exceptions or drawbacks and limitations. In some compounds such as \[LiCl\], \[Be{H_2}\], \[AlC{l_3}\], etc., the number of electrons surrounding the central atom (whose valence electrons are less than four) is less than eight after the bonding. Also, in molecules with an odd number of electrons like \[NO\] and \[N{O_2}\], the octet rule is not satisfied for all the atoms. Furthermore, some compounds such as PF5, there are more than eight valence electrons around the central atom (expanded octet).Other drawbacks of the octet rule are:
(i) This theory is not helpful for the prediction of shape of molecules.
(ii) Although octet rule is based on the chemical inertness of noble gases, some noble gases do combine with oxygen and fluorine to form compounds such as $XeOF_3$.
(iii) This rule does not explain the relative stability of molecules.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




