
Explain the formation of ${\text{M}}{{\text{g}}_3}{{\text{N}}_2}$
Answer
564.6k+ views
Hint:A chemical bond is formed between two atoms of different or same elements by either sharing of electrons (covalent bond), or between two atoms having a large difference in their electron affinity (ionic bonding).
Complete step by step solution:
Now to identify the formation of in ${\text{M}}{{\text{g}}_3}{{\text{N}}_2}$ we will look at their respective valence i.e., the number of electrons in their outermost orbitals or the combining power of the element.
Magnesium is a group 2 element, and the valence of the element is +2.
This gives us the valence of the 3 Mg atom as +6.
And the valence of one nitrogen atom is -3, which implies the valence of 2 nitrogen atom is -6
We can see that ${\text{M}}{{\text{g}}_3}{{\text{N}}_2}$ is a electrically neutral molecule, and the -6 valence of 2 nitrogen and +6 valence of 3 magnesium guarantee this statement.
The molecular formula of ${\text{M}}{{\text{g}}_3}{{\text{N}}_2}$ can be written by following the criss cross valence:
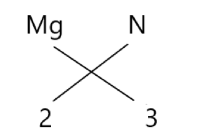
Hence, the formation of ${\text{M}}{{\text{g}}_3}{{\text{N}}_2}$ occurs when 6 electrons of are donated from 3 magnesium to 2 nitrogens, this results in the formation of a ionic bond between them.
Additional information:Steps to use in determining the molecular formula of diatomic molecules using criss cross valence,
i. Identify the valence of each atom using the knowledge of periodic table
ii. Exchange the valence of the atoms with each other
iii. Reduce the valence number to the lowest ratio, if possible
iv. Write the chemical formula.
Let’s assume that we have 2 atoms X and Y, having valence 2 and 4 respectively, using criss cross valence we can write the molecular formula as ${{\text{X}}_4}{{\text{Y}}_2}$ . But the valence of the atoms can be reduced the lowest ratio 1 : 2 (i.e, 1 for atom X and 2 from atom Y) now after criss crossing the molecular formula can be written as ${{\text{X}}_2}{{\text{Y}}_1}$.
Note:The numbers written under magnesium and nitrogen (i.e., 3 and 2 respectively) represents the number of atoms of magnesium and nitrogen present in one ${\text{M}}{{\text{g}}_3}{{\text{N}}_2}$ molecule and not the respective valence of these atoms.
Complete step by step solution:
Now to identify the formation of in ${\text{M}}{{\text{g}}_3}{{\text{N}}_2}$ we will look at their respective valence i.e., the number of electrons in their outermost orbitals or the combining power of the element.
Magnesium is a group 2 element, and the valence of the element is +2.
This gives us the valence of the 3 Mg atom as +6.
And the valence of one nitrogen atom is -3, which implies the valence of 2 nitrogen atom is -6
We can see that ${\text{M}}{{\text{g}}_3}{{\text{N}}_2}$ is a electrically neutral molecule, and the -6 valence of 2 nitrogen and +6 valence of 3 magnesium guarantee this statement.
The molecular formula of ${\text{M}}{{\text{g}}_3}{{\text{N}}_2}$ can be written by following the criss cross valence:

Hence, the formation of ${\text{M}}{{\text{g}}_3}{{\text{N}}_2}$ occurs when 6 electrons of are donated from 3 magnesium to 2 nitrogens, this results in the formation of a ionic bond between them.
Additional information:Steps to use in determining the molecular formula of diatomic molecules using criss cross valence,
i. Identify the valence of each atom using the knowledge of periodic table
ii. Exchange the valence of the atoms with each other
iii. Reduce the valence number to the lowest ratio, if possible
iv. Write the chemical formula.
Let’s assume that we have 2 atoms X and Y, having valence 2 and 4 respectively, using criss cross valence we can write the molecular formula as ${{\text{X}}_4}{{\text{Y}}_2}$ . But the valence of the atoms can be reduced the lowest ratio 1 : 2 (i.e, 1 for atom X and 2 from atom Y) now after criss crossing the molecular formula can be written as ${{\text{X}}_2}{{\text{Y}}_1}$.
Note:The numbers written under magnesium and nitrogen (i.e., 3 and 2 respectively) represents the number of atoms of magnesium and nitrogen present in one ${\text{M}}{{\text{g}}_3}{{\text{N}}_2}$ molecule and not the respective valence of these atoms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




