
Explain the formation of the $B{{F}_{3}}$ molecule using hybridisation.
Answer
546k+ views
Hint: We know that hybridization is the mixing of atomic orbitals into new hybrid orbitals. The many types of hybridisation includes $s{{p}^{3}}$, $s{{p}^{2}}$ and sp.
Complete answer:
$\text{B}{{\text{F}}_{\text{3}}}$ molecule has a geometrical shape of “Trigonal Planar”. The bond angle made was ${{120}^{\circ }}$ with Boron at the centre and Fluorine at the 3 vertices forming an equilateral triangle. In case of $\text{B}{{\text{F}}_{\text{3}}}$, the central atom does not have an octet as it has 6 particles. It becomes an octet after addition of more bonds. The structure can be drawn as:
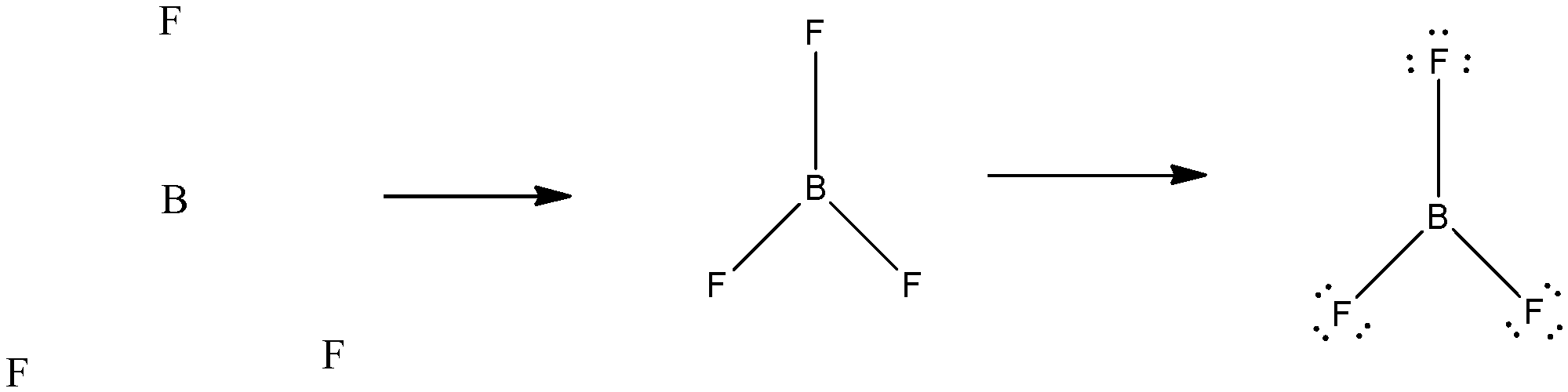
Since, a pi ( $\pi $ ) bond is required for double bond between boron and 3 sigma ($\text{ }\!\!\sigma\!\!\text{ }$) bonds are formed per atom of boron, $B{{F}_{3}}$is $s{{p}^{2}}$ hybridised. The atomic S-orbitals and P-orbitals in the outer shell of Boron mix to form three equivalent $s{{p}^{2}}$ hybrid orbitals.
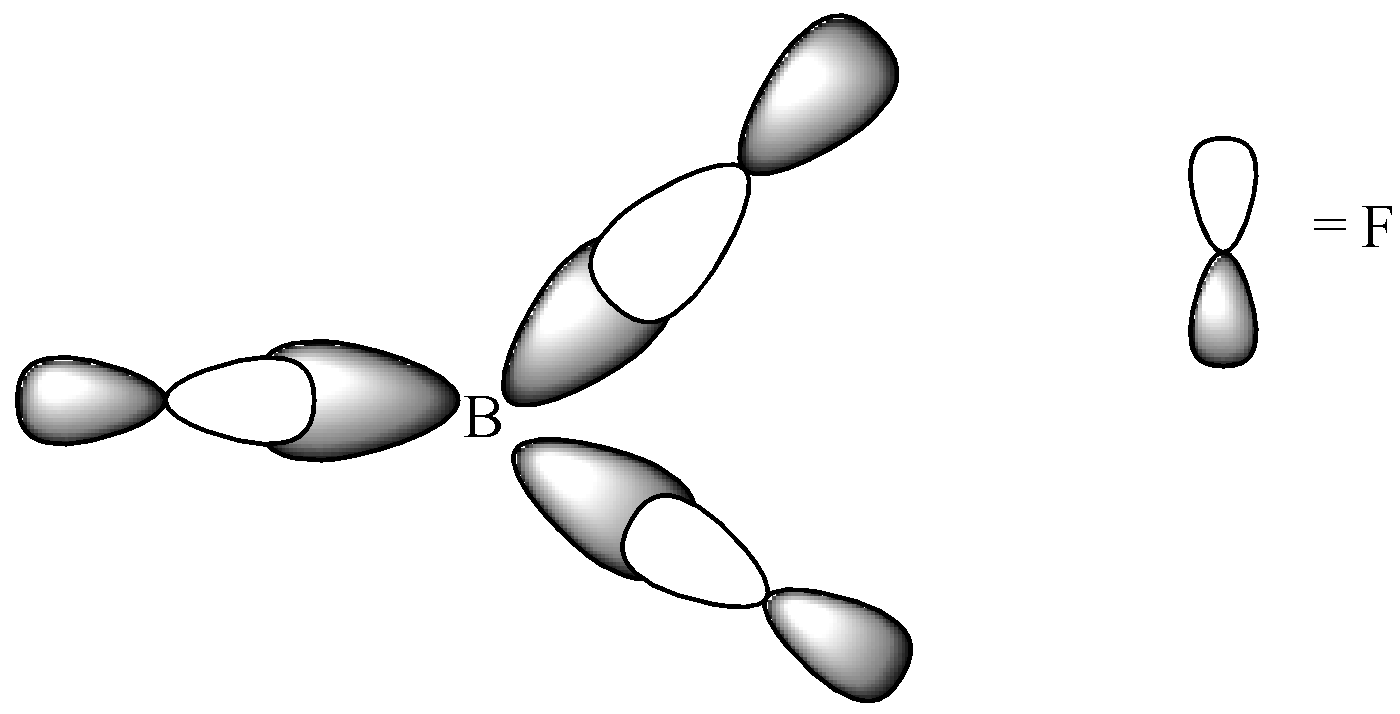
We can see that Boron has an electronic configuration \[\text{1}{{\text{s}}^{\text{2}}}\text{2}{{\text{s}}^{\text{2}}}\text{2}{{\text{p}}_{\text{x}}}^{\text{1}}\] . When it is excited, the electronic configuration changes into \[\text{1}{{\text{s}}^{\text{2}}}\text{ 2}{{\text{s}}^{\text{1}}}\text{ 2p}_{x}^{1}\text{ 2p}_{y}^{1}\] . Three identical B-F bonds are created in $\text{B}{{\text{F}}_{\text{3}}}$ and the excited boron atom undergoes hybridisation. The intermixing and redistribution of 2s, $2{{p}_{x}}$ and $2{{p}_{y}}$ orbitals into three identical orbits form $s{{p}^{2}}$ hybrid orbitals. For minimum repulsion to be present, the angle between two orbitals in the molecule must be ${{120}^{\circ }}$. Each $s{{p}^{2}}$ orbital must receive an electron and then 3 fluorine atoms overlap their $2{{p}_{z}}$ orbitals containing unpaired electrons. Finally, the three $s{{p}^{2}}$ orbitals of boron atoms contained unpaired electrons and formed three sigma-$s{{p}^{2}}$ -p bonds.
Note:
We should know that boron trifluoride is electron poor and has an empty orbital. This enables it to accept a pair of electrons. Thus we can say that it is a Lewis Acid.
Complete answer:
$\text{B}{{\text{F}}_{\text{3}}}$ molecule has a geometrical shape of “Trigonal Planar”. The bond angle made was ${{120}^{\circ }}$ with Boron at the centre and Fluorine at the 3 vertices forming an equilateral triangle. In case of $\text{B}{{\text{F}}_{\text{3}}}$, the central atom does not have an octet as it has 6 particles. It becomes an octet after addition of more bonds. The structure can be drawn as:

Since, a pi ( $\pi $ ) bond is required for double bond between boron and 3 sigma ($\text{ }\!\!\sigma\!\!\text{ }$) bonds are formed per atom of boron, $B{{F}_{3}}$is $s{{p}^{2}}$ hybridised. The atomic S-orbitals and P-orbitals in the outer shell of Boron mix to form three equivalent $s{{p}^{2}}$ hybrid orbitals.

We can see that Boron has an electronic configuration \[\text{1}{{\text{s}}^{\text{2}}}\text{2}{{\text{s}}^{\text{2}}}\text{2}{{\text{p}}_{\text{x}}}^{\text{1}}\] . When it is excited, the electronic configuration changes into \[\text{1}{{\text{s}}^{\text{2}}}\text{ 2}{{\text{s}}^{\text{1}}}\text{ 2p}_{x}^{1}\text{ 2p}_{y}^{1}\] . Three identical B-F bonds are created in $\text{B}{{\text{F}}_{\text{3}}}$ and the excited boron atom undergoes hybridisation. The intermixing and redistribution of 2s, $2{{p}_{x}}$ and $2{{p}_{y}}$ orbitals into three identical orbits form $s{{p}^{2}}$ hybrid orbitals. For minimum repulsion to be present, the angle between two orbitals in the molecule must be ${{120}^{\circ }}$. Each $s{{p}^{2}}$ orbital must receive an electron and then 3 fluorine atoms overlap their $2{{p}_{z}}$ orbitals containing unpaired electrons. Finally, the three $s{{p}^{2}}$ orbitals of boron atoms contained unpaired electrons and formed three sigma-$s{{p}^{2}}$ -p bonds.
Note:
We should know that boron trifluoride is electron poor and has an empty orbital. This enables it to accept a pair of electrons. Thus we can say that it is a Lewis Acid.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




