
How would you explain the halogenation of benzene?
Answer
534k+ views
Hint :Halogenation is the type of reaction in which we observe the replacement of the halogen atom with another substance where we see the halogen atom ends up as a part of the substance or the compound.
Complete Step By Step Answer:
The aromatic compounds undergo the electrophilic halogenation during the presence of Lewis acids. So the compound benzene usually reacts with bromine or the halogen chlorine in the electrophilic substitution reaction when a catalyst is present and that catalyst is either the chloride or iron. As we know that irons cannot be considered as a catalyst because it tends to change permanently in the reaction. So it tends to react with chlorine to form iron(III) chloride or bromine to form iron (III) bromide.
We see that the halogen bromine reacts with the Lewis acid so that the formation of the complex takes place that makes the halogen bromine more electrophile.
We see that the pi electrons of the aromatic $ C=C $ behave as a nucleophile which ten attacks the electrophile bromine and then displaces the iron tetra bromide.
We see the proton is getting removed from the $ s{{p}^{3}} $ hybridized carbon atoms and bears the group of bromo which reforms the carbon carbon double bond and the aromatic system tends to generate the HBr and tends to regulate the active catalyst. The compounds tend to act as a catalyst and behave in the similar manner of aluminium chloride.
When the benzene is reacted with chlorine under the presence of aluminium chloride the formation of chlorobenzene takes place. Generally we observe in the reaction that one or more halogens are added to the substance. There are different types of halogenation reactions such as halogen addition, halogen substitution and electrophilic substitution reaction.
Electrophilic aromatic substitution is a reaction in which an atom on an aromatic ring is replaced by an electrophile. A typical halogenation reaction is:
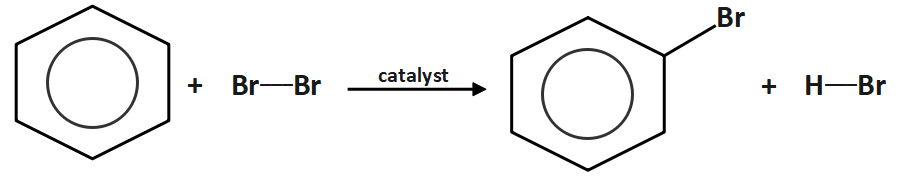
The electrophile is an ion that is generated by the catalyst.
Note :
When we react the benzene with the bromine under the presence of aluminium bromide the formation of bromobenzene and hydrogen bromide takes place. But if we react it with elemental fluorine then we need some proper apparatus and need to fulfil certain conditions. And if we react benzene with iodine nothing is formed as iodine does not react readily with the organic compounds.
Complete Step By Step Answer:
The aromatic compounds undergo the electrophilic halogenation during the presence of Lewis acids. So the compound benzene usually reacts with bromine or the halogen chlorine in the electrophilic substitution reaction when a catalyst is present and that catalyst is either the chloride or iron. As we know that irons cannot be considered as a catalyst because it tends to change permanently in the reaction. So it tends to react with chlorine to form iron(III) chloride or bromine to form iron (III) bromide.
We see that the halogen bromine reacts with the Lewis acid so that the formation of the complex takes place that makes the halogen bromine more electrophile.
We see that the pi electrons of the aromatic $ C=C $ behave as a nucleophile which ten attacks the electrophile bromine and then displaces the iron tetra bromide.
We see the proton is getting removed from the $ s{{p}^{3}} $ hybridized carbon atoms and bears the group of bromo which reforms the carbon carbon double bond and the aromatic system tends to generate the HBr and tends to regulate the active catalyst. The compounds tend to act as a catalyst and behave in the similar manner of aluminium chloride.
When the benzene is reacted with chlorine under the presence of aluminium chloride the formation of chlorobenzene takes place. Generally we observe in the reaction that one or more halogens are added to the substance. There are different types of halogenation reactions such as halogen addition, halogen substitution and electrophilic substitution reaction.
Electrophilic aromatic substitution is a reaction in which an atom on an aromatic ring is replaced by an electrophile. A typical halogenation reaction is:

The electrophile is an ion that is generated by the catalyst.
Note :
When we react the benzene with the bromine under the presence of aluminium bromide the formation of bromobenzene and hydrogen bromide takes place. But if we react it with elemental fluorine then we need some proper apparatus and need to fulfil certain conditions. And if we react benzene with iodine nothing is formed as iodine does not react readily with the organic compounds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




