
Explain the molecular orbital structure, bond order, stability and magnetic behaviour of Hydrogen molecules on the basis of molecular orbital theory.
Answer
583.8k+ views
Hint: Molecular Orbital Theory, abbreviated as MOT is a theory on chemical bonding developed by F.Hund and R.S. Mulliken at the beginning of the twentieth century to describe the structure and properties of different molecules.
Complete step by step solution:
-Since the valence-bond theory failed to satisfactorily explain how certain molecules contain two or more equivalent bonds whose bond orders lie between that of a single bond and that of a double bond, such as the bonds in resonance-stabilized molecules, the molecular orbital theory proved to be powerful as it explained all these limitations of valence bond theory.
-Following are the key features of the Molecular Orbital Theory-
(i) The total number of molecular orbitals formed will always be equal to the total number of atomic orbitals provided by the bonding species.
(ii) Among three different types of molecular orbitals, (viz. bonding molecular orbitals, anti-bonding molecular orbitals, and non-bonding molecular orbitals) anti-bonding molecular orbitals will always have higher energy than the parent’s orbitals whereas bonding molecular orbitals will always have lower energy than the parent orbitals.
(iii) The filling of electrons into molecular orbitals follows Aufbau principle, Pauli’s Exclusion principle, and Hund’s rule of maximum multiplicity, which says that filling of electrons will occur in the order of orbital energy (from the orbital with the lowest orbital to the orbital with the highest energy).
(iv) When the combining of atomic orbitals of similar energies occurs, the most effective combinations are formed.
-In simple words, the Molecular Orbital Theory states that each atom tends to combine with other atomic orbitals to form molecular orbitals. As a result of such rearrangements, electrons which are associated with different nuclei are found in various atomic orbitals.
-The difference between the number of bonds and antibonds is defined by bond order. The bond number is the number of electron pairs (bonds) present between a pair of atoms. Bond order gives the indication of the stability of a bond. In molecular orbital theory, the bond order is defined as the half the difference between the number of electrons in bonding orbitals and the number of electrons in antibonding orbitals.
$\text{Bond order}=\dfrac{\text{Bonding electrons - Nonbonding electrons}}{2}$
-In case of the Hydrogen molecule, there are only two bonding electrons,
Therefore, $\text{Bond order}=\dfrac{2-0}{2}=1$
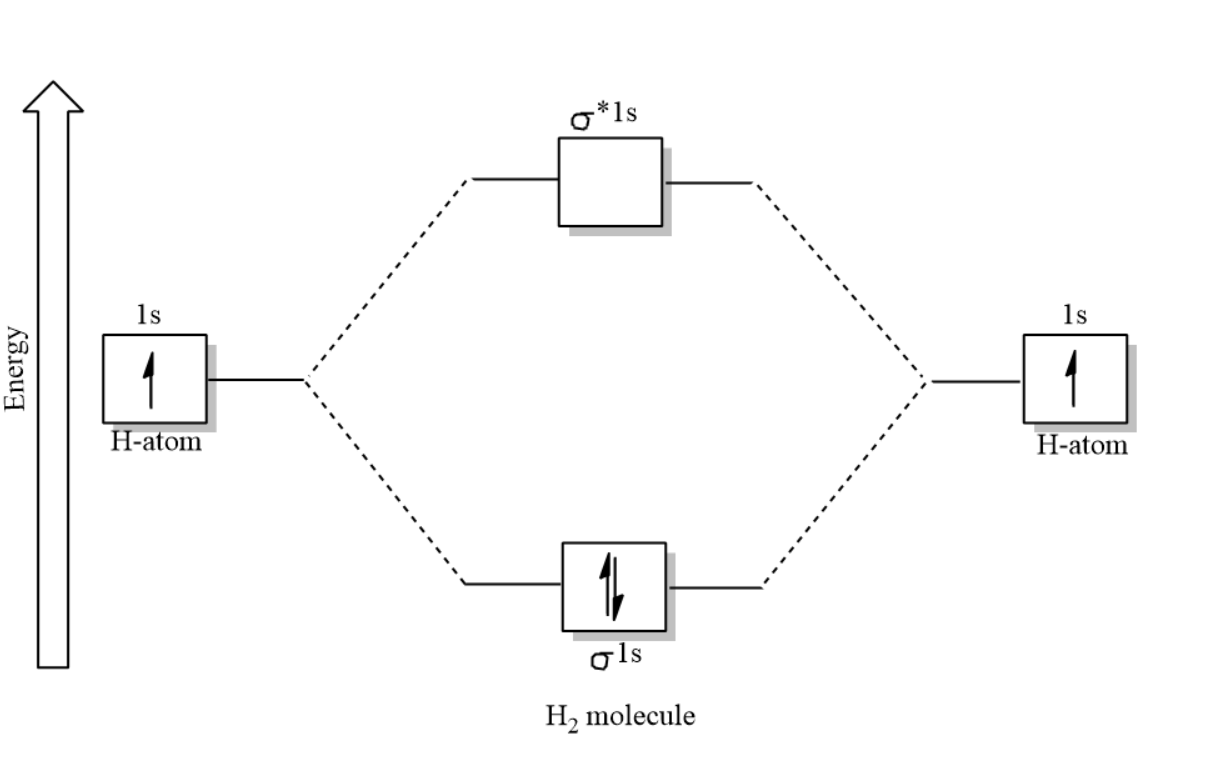
Since there are no unpaired electrons present, the hydrogen molecule is stable and Diamagnetic.
Note: Molecular Orbital Theory has limitations too, which are given below-
(i) Molecular orbital explains whether the molecule will exist or not on the basis of bond order. According to MOT, if the bond order comes out to be 1, it implies that the molecule must exist and have a single bond in it, but no such molecule exists practically.
(ii) Concept of bond order used in MOT was not feasible and appropriate to explain the existence of polyatomic molecules.
(iii) MOT does not give any idea about molecular geometry and the shape of the molecule.
Complete step by step solution:
-Since the valence-bond theory failed to satisfactorily explain how certain molecules contain two or more equivalent bonds whose bond orders lie between that of a single bond and that of a double bond, such as the bonds in resonance-stabilized molecules, the molecular orbital theory proved to be powerful as it explained all these limitations of valence bond theory.
-Following are the key features of the Molecular Orbital Theory-
(i) The total number of molecular orbitals formed will always be equal to the total number of atomic orbitals provided by the bonding species.
(ii) Among three different types of molecular orbitals, (viz. bonding molecular orbitals, anti-bonding molecular orbitals, and non-bonding molecular orbitals) anti-bonding molecular orbitals will always have higher energy than the parent’s orbitals whereas bonding molecular orbitals will always have lower energy than the parent orbitals.
(iii) The filling of electrons into molecular orbitals follows Aufbau principle, Pauli’s Exclusion principle, and Hund’s rule of maximum multiplicity, which says that filling of electrons will occur in the order of orbital energy (from the orbital with the lowest orbital to the orbital with the highest energy).
(iv) When the combining of atomic orbitals of similar energies occurs, the most effective combinations are formed.
-In simple words, the Molecular Orbital Theory states that each atom tends to combine with other atomic orbitals to form molecular orbitals. As a result of such rearrangements, electrons which are associated with different nuclei are found in various atomic orbitals.
-The difference between the number of bonds and antibonds is defined by bond order. The bond number is the number of electron pairs (bonds) present between a pair of atoms. Bond order gives the indication of the stability of a bond. In molecular orbital theory, the bond order is defined as the half the difference between the number of electrons in bonding orbitals and the number of electrons in antibonding orbitals.
$\text{Bond order}=\dfrac{\text{Bonding electrons - Nonbonding electrons}}{2}$
-In case of the Hydrogen molecule, there are only two bonding electrons,
Therefore, $\text{Bond order}=\dfrac{2-0}{2}=1$

Since there are no unpaired electrons present, the hydrogen molecule is stable and Diamagnetic.
Note: Molecular Orbital Theory has limitations too, which are given below-
(i) Molecular orbital explains whether the molecule will exist or not on the basis of bond order. According to MOT, if the bond order comes out to be 1, it implies that the molecule must exist and have a single bond in it, but no such molecule exists practically.
(ii) Concept of bond order used in MOT was not feasible and appropriate to explain the existence of polyatomic molecules.
(iii) MOT does not give any idea about molecular geometry and the shape of the molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




