
How would you explain the phase diagram of sulfur?
Answer
560.7k+ views
Hint: The phase diagram of an element or a compound tells the inter-conversion properties or relation between the solid phase, liquid phase, and gas phase of that substance. There is one gas phase, one liquid phase, and two solid phases of sulfur. The graph is drawn between the temperature and pressure.
Complete step by step answer:
- The phase diagram of an element or a compound tells the inter-conversion properties or relation between the solid phase, liquid phase, and gas phase of that substance. The graph is drawn between the temperature and pressure.
- Sulfur is the element of group 16 of the p-block and it is the element of period 2. There is one gas phase, one liquid phase, and two solid phases of sulfur. The two solid phases of the sulfur atom are monoclinic and rhombic form. The phase diagram of sulfur is given below:
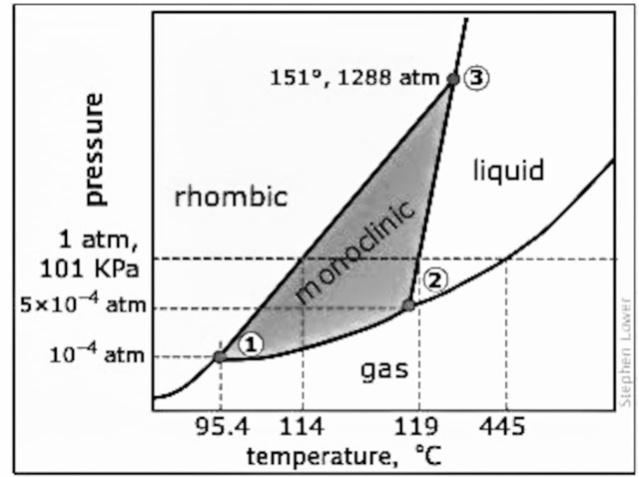
- There are three triple points of sulfur and it is indicated in the diagram with numbers 1, 2, and 3. The most stable form of a sulfur element is the rhombic form and when it is heated slowly then the rhombic form will convert into monoclinic form at temperature around ${{114}^{\circ }}C$. This solid form will convert into liquid form at $119-{{120}^{\circ }}C$ because the solid form melts at this temperature. As the pressure decreases the gas form dominates. At the first triple point the rhombic solid, monoclinic solid, and the gas form co-exist and the temperature is ${{95.31}^{\circ }}C$ at $5.1\text{ x 1}{{\text{0}}^{-6}}$ atm. At the second triple point, the monoclinic solid, the gas form, and the liquid form co-exists and the temperature is ${{115.18}^{\circ }}C$ at $3.2\text{ x 1}{{\text{0}}^{-5}}$ atm. At the third triple point, the monoclinic solid form, the rhombic solid form, and the liquid form co-exist and the temperature is ${{153}^{\circ }}C$ at 1420 atm.
Note: The elemental form of sulfur is ${{S}_{8}}$ which means that there are 8 atoms of sulfur in one molecule. More than one solid form is not common and when there is more than one solid form the phase diagram becomes a little complicated.
Complete step by step answer:
- The phase diagram of an element or a compound tells the inter-conversion properties or relation between the solid phase, liquid phase, and gas phase of that substance. The graph is drawn between the temperature and pressure.
- Sulfur is the element of group 16 of the p-block and it is the element of period 2. There is one gas phase, one liquid phase, and two solid phases of sulfur. The two solid phases of the sulfur atom are monoclinic and rhombic form. The phase diagram of sulfur is given below:

- There are three triple points of sulfur and it is indicated in the diagram with numbers 1, 2, and 3. The most stable form of a sulfur element is the rhombic form and when it is heated slowly then the rhombic form will convert into monoclinic form at temperature around ${{114}^{\circ }}C$. This solid form will convert into liquid form at $119-{{120}^{\circ }}C$ because the solid form melts at this temperature. As the pressure decreases the gas form dominates. At the first triple point the rhombic solid, monoclinic solid, and the gas form co-exist and the temperature is ${{95.31}^{\circ }}C$ at $5.1\text{ x 1}{{\text{0}}^{-6}}$ atm. At the second triple point, the monoclinic solid, the gas form, and the liquid form co-exists and the temperature is ${{115.18}^{\circ }}C$ at $3.2\text{ x 1}{{\text{0}}^{-5}}$ atm. At the third triple point, the monoclinic solid form, the rhombic solid form, and the liquid form co-exist and the temperature is ${{153}^{\circ }}C$ at 1420 atm.
Note: The elemental form of sulfur is ${{S}_{8}}$ which means that there are 8 atoms of sulfur in one molecule. More than one solid form is not common and when there is more than one solid form the phase diagram becomes a little complicated.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




