
Explain the preparation of phenol from cumene.
Answer
596.1k+ views
Hint:We get two products from this reaction. One of them is a ketone and the other one is an alcohol.
Complete step by step solution:
Let’s talk about cumene first. Its scientific name is isopropyl benzene. It is a natural by-product of the extraction of crude oil and coal tar which are petroleum products. Cumene is a colourless and volatile liquid which has a smell similar to gasoline. It is used in industries for the production of phenol on a large scale. The structure of cumene is as below:
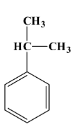
The reaction of cumene that produces phenol is as shown below:
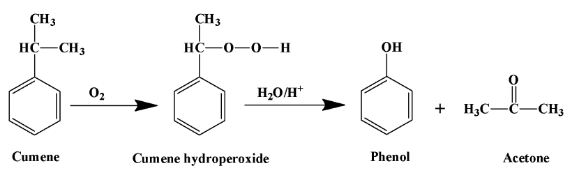
Taking the above reaction in a stepwise manner is good for health. It is as explained below:
- Cumene is first oxidised into cumene hydroperoxide by exposing it to air. This process is called air oxidation as no specific reagent is added to the reaction mixture for oxidising cumene. The product is a peroxide which means it has an “$O-O$” bond. This process follows a free-radical mechanism.
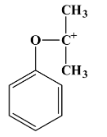
- The degradation of cumene hydroperoxide occurs through hydrolysis in an acidic medium. The mechanism in this case involves a carbocation intermediate. The carbocation is tertiary in nature and has the following structure:
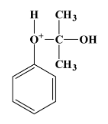
- The carbocation molecule is then attacked by another water molecule, which gives the following product:
- The positive charge on the oxygen atom directly attached to the benzene ring is due to the bonding of oxygen to three atoms while its valency is only two. This creates instability in the structure. The bond between ${{O}^{+}}-C$ is cleaved by the actions of the acidic medium and we get our final products as phenol and acetone.
Note:
- This reaction is only possible in an acidic medium. $\text{Propan}-2-ol$ produced in the second last step is oxidised into acetone only because of the presence of an acidic medium.
- This reaction is mainly used in industries. Replicating the same in laboratories is not possible because of the air oxidation process that requires heavy and complex equipment.
Complete step by step solution:
Let’s talk about cumene first. Its scientific name is isopropyl benzene. It is a natural by-product of the extraction of crude oil and coal tar which are petroleum products. Cumene is a colourless and volatile liquid which has a smell similar to gasoline. It is used in industries for the production of phenol on a large scale. The structure of cumene is as below:

The reaction of cumene that produces phenol is as shown below:

Taking the above reaction in a stepwise manner is good for health. It is as explained below:
- Cumene is first oxidised into cumene hydroperoxide by exposing it to air. This process is called air oxidation as no specific reagent is added to the reaction mixture for oxidising cumene. The product is a peroxide which means it has an “$O-O$” bond. This process follows a free-radical mechanism.
- The degradation of cumene hydroperoxide occurs through hydrolysis in an acidic medium. The mechanism in this case involves a carbocation intermediate. The carbocation is tertiary in nature and has the following structure:

- The carbocation molecule is then attacked by another water molecule, which gives the following product:

- The positive charge on the oxygen atom directly attached to the benzene ring is due to the bonding of oxygen to three atoms while its valency is only two. This creates instability in the structure. The bond between ${{O}^{+}}-C$ is cleaved by the actions of the acidic medium and we get our final products as phenol and acetone.
Note:
- This reaction is only possible in an acidic medium. $\text{Propan}-2-ol$ produced in the second last step is oxidised into acetone only because of the presence of an acidic medium.
- This reaction is mainly used in industries. Replicating the same in laboratories is not possible because of the air oxidation process that requires heavy and complex equipment.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




