
Explain the process of getting pure salt from impure salt with a diagram.
Answer
551.7k+ views
Hint: To answer this question, you must recall the process involved in the separation of pure salt from a given mixture of the salt and its impurities. There are many ways of separating a salt from its impurities namely, crystallization, sublimation, filtration, etc. depending upon the nature of the salt and the impurities.
Complete step by step solution:
The method of separation of a salt from impurities is selected on the basis of the property of both the impurity as well as the salt. If the impurities in the impure salt mixture are not soluble in water and the salt is soluble, then the pure salt can be obtained by the process of filtration. The process is done as follows:
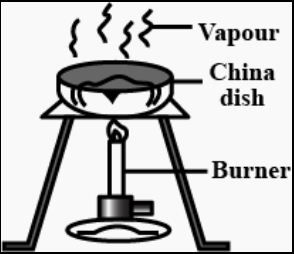
First we add some water in a china dish or a beaker. Then we add the impure salt in it and stir it well so as to dissolve the salt. The impurities however being insoluble would settle down in the solution. This sediment can be separated from the mixture by filtration. Now we have a pure salt solution, from which we can get the pure desired salt by evaporating all the water by heating the beaker or china dish.
Note:
If the salt we have to purify is a sublime salt, then we can get the pure salt by sublimation and then collecting the vapours and crystallizing them. If both the salt and the impurities are soluble in a particular solvent, the salt can be purified by the process of crystallization, in which the crystals of pure salt crystallize, separating the pure salt from the rest of the impure solution.
Complete step by step solution:
The method of separation of a salt from impurities is selected on the basis of the property of both the impurity as well as the salt. If the impurities in the impure salt mixture are not soluble in water and the salt is soluble, then the pure salt can be obtained by the process of filtration. The process is done as follows:
First we add some water in a china dish or a beaker. Then we add the impure salt in it and stir it well so as to dissolve the salt. The impurities however being insoluble would settle down in the solution. This sediment can be separated from the mixture by filtration. Now we have a pure salt solution, from which we can get the pure desired salt by evaporating all the water by heating the beaker or china dish.

Note:
If the salt we have to purify is a sublime salt, then we can get the pure salt by sublimation and then collecting the vapours and crystallizing them. If both the salt and the impurities are soluble in a particular solvent, the salt can be purified by the process of crystallization, in which the crystals of pure salt crystallize, separating the pure salt from the rest of the impure solution.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




