
Explain the structure of ammonia.
Answer
598.8k+ views
Hint: The structure of any compound is determined by the VSEPR theory. It determines the type of hybridization present in a compound and its shape.
Complete answer step by step:
Ammonia is a colorless gas having a distinct odor. It is composed of nitrogen and hydrogen atoms. The chemical formula of ammonia is $N{H}_{3}$. The structure of ammonia is defined by the VSEPR and valence bond theory. VSEPR is an acronym for the Valence Shell Electron Pair Repulsion theory. This theory is used to predict the shape of the molecules from the electron pairs that surround the central atom. According to this theory,
$H\quad =\quad \cfrac { 1 }{ 2 } \left[ V+M-C+A \right]$
where,
H = Number of orbitals involved in hybridization.
V = Valence electrons of the central atom.
M = Number of monovalent atoms linked to the central atom.
C = Charge of the cation.
A = Charge of the anion.
In case of ammonia $(N{H}_{3})$, V=5, M=3, C=0 and A=0. Substituting these values in the above equation, we get
$H\quad =\quad \cfrac { 1 }{ 2 } (5\quad +\quad 3)$
$\implies H = 4$
This means that the no. of orbitals or electron pairs involved are 4. Therefore, the hybridization of $(N{H}_{3})$ is $s{p}^{3}$. But since it has only three atoms of hydrogen, one position will be occupied by a lone pair.
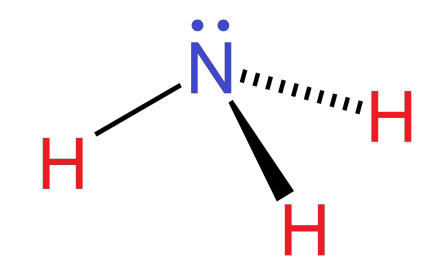
Therefore, ammonia has a trigonal pyramidal shape according to the VSEPR theory. It has an experimental bond angle of $106.{7}^{0}$.
Note: In the above-mentioned case, due to $s{p}^{3}$ hybridization, the shape of ammonia should have been tetrahedral. But due to the presence of only 3 hydrogen atoms and a lone pair, its shape is trigonal pyramidal.
Complete answer step by step:
Ammonia is a colorless gas having a distinct odor. It is composed of nitrogen and hydrogen atoms. The chemical formula of ammonia is $N{H}_{3}$. The structure of ammonia is defined by the VSEPR and valence bond theory. VSEPR is an acronym for the Valence Shell Electron Pair Repulsion theory. This theory is used to predict the shape of the molecules from the electron pairs that surround the central atom. According to this theory,
$H\quad =\quad \cfrac { 1 }{ 2 } \left[ V+M-C+A \right]$
where,
H = Number of orbitals involved in hybridization.
V = Valence electrons of the central atom.
M = Number of monovalent atoms linked to the central atom.
C = Charge of the cation.
A = Charge of the anion.
In case of ammonia $(N{H}_{3})$, V=5, M=3, C=0 and A=0. Substituting these values in the above equation, we get
$H\quad =\quad \cfrac { 1 }{ 2 } (5\quad +\quad 3)$
$\implies H = 4$
This means that the no. of orbitals or electron pairs involved are 4. Therefore, the hybridization of $(N{H}_{3})$ is $s{p}^{3}$. But since it has only three atoms of hydrogen, one position will be occupied by a lone pair.

Therefore, ammonia has a trigonal pyramidal shape according to the VSEPR theory. It has an experimental bond angle of $106.{7}^{0}$.
Note: In the above-mentioned case, due to $s{p}^{3}$ hybridization, the shape of ammonia should have been tetrahedral. But due to the presence of only 3 hydrogen atoms and a lone pair, its shape is trigonal pyramidal.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




