
Explain the structure of diborane .
Answer
564.9k+ views
Hint: Diborane is colourless having chemical formula of ${B_2}{H_6}$.it is toxic in case inhaled. It has an offensive odor. Diborane is formed by boric acid and water. It is easily mixed with air and becomes explosive . so, it must be handled carefully .
Complete answer:
Diborane is made up of a total eight hydrogen atoms and two borons . In which two hydrogen are on the same plane. While the other two hydrogen forms a bridge. Bridge hydrogens are above and below the plane. These bonds are also called banana bonds . The hybridisation of Diborane is $s{p^3}$ in which the length of $B - {H_{bridge}}$ is $1.33$ and length of $B - {H_{ter\min al}}$ is 1.19 $\mathop A\limits^ \circ $.
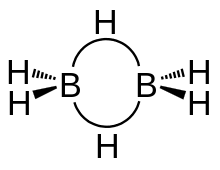
in the diborane the two boron do not form any bonds . The angle between the two terminal hydrogen and one boron is ${120^ \circ }$ and the angle between the bridge hydrogen and
boron is ${97^ \circ }$
Additional information:
Borane can be formed by the reaction of sodium tetrahydridoborate and boron trifluoride etherate . This method is considered the most convenient process for manufacturing diborane .
it can't exist as $B{H_3}$ because boron has three electrons in its valence shell . It is an electron deficient compound and unstable . As after forming a bond with three hydrogens the total number of electrons around the boron is six so it is considered as electron deficient and thus it exists as a dimer which we call diborane .
Note:
Diborane is used in rubber vulcaniser , rocket propellants and a doping agent in the manufacture of semiconductors.It is also used as a reducing agent. It releases a huge amount of energy when burnt in oxygen.
Complete answer:
Diborane is made up of a total eight hydrogen atoms and two borons . In which two hydrogen are on the same plane. While the other two hydrogen forms a bridge. Bridge hydrogens are above and below the plane. These bonds are also called banana bonds . The hybridisation of Diborane is $s{p^3}$ in which the length of $B - {H_{bridge}}$ is $1.33$ and length of $B - {H_{ter\min al}}$ is 1.19 $\mathop A\limits^ \circ $.

in the diborane the two boron do not form any bonds . The angle between the two terminal hydrogen and one boron is ${120^ \circ }$ and the angle between the bridge hydrogen and
boron is ${97^ \circ }$
Additional information:
Borane can be formed by the reaction of sodium tetrahydridoborate and boron trifluoride etherate . This method is considered the most convenient process for manufacturing diborane .
it can't exist as $B{H_3}$ because boron has three electrons in its valence shell . It is an electron deficient compound and unstable . As after forming a bond with three hydrogens the total number of electrons around the boron is six so it is considered as electron deficient and thus it exists as a dimer which we call diborane .
Note:
Diborane is used in rubber vulcaniser , rocket propellants and a doping agent in the manufacture of semiconductors.It is also used as a reducing agent. It releases a huge amount of energy when burnt in oxygen.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




