
Explain why a sigma (σ) bond is stronger than a pi (π) bond?
Answer
527.7k+ views
Hint: sigma bond is formed by the head to head overlap of orbitals. The pi bond is formed by the lateral or sideway overlapping of orbitals. The strength of bond depends on the bond length. Higher the bond length higher is the energy required for the bond breaking.
Complete step by step answer:
The covalent bond is formed by the head to head overlap of atomic orbitals. A covalent bond formed is linear or coaxial. The bond formed is in a line internuclear axis of atomic orbitals. This is known as a sigma bond. For example, the s-orbitals undergo the head to head overlap to form a sigma bond as shown.
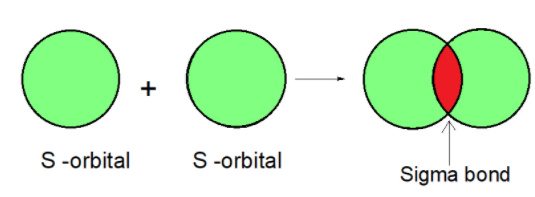
The covalent bond is formed on the internuclear axis. Let’s consider the example of s orbitals. S-orbitals are spherical and can overlap from any side. The S orbital overlap forms a sigma bond.
1) The Sigma bond is covalent which is formed by the overlap of atomic orbitals.
2) It is possible between two orbitals such as s-s, p-p, or s-p.
3) They have high bond energy.
-A covalent bond that is formed by the lateral or sideway overlapping of pure orbitals is called the pi bond. It is formed by the side to side overlapping of orbitals. There is three types of p orbitals${{P}_{x}},{{P}_{y}}\text{ and }{{\text{P}}_{\text{z}}}$. This is mutually perpendicular to each other. The pi bond is between the p orbitals which are mutually parallel to each other but perpendicular to the internuclear axis. For example, the orbitals ${{p}_{y}}-{{p}_{y}}$overlap to form a pi bond as shown.
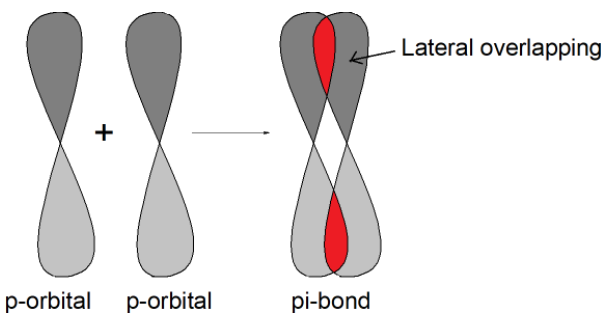
1) This is formed by the sideway overlap of atomic orbitals.
2) It exists between two p orbital only.
3) Have less bond energy than sigma bonds.
4) It forms only when the molecule has already formed a sigma bond.
5) It is weak due to the lower extent of overlapping.
Here, the extent of overlapping of the orbital along the internuclear axis is greater than the sideway overlapping of atomic orbitals. We know that, the larger the extent of overlapping the stronger the bond formed. In sigma bonds, the large overlap of the orbital involves the removal of a large amount of energy. While in pi bonds the extent of overlapping is less than sigma bond.
Therefore, sigma bond is stronger than pi bond.
Note: Bond strength depends on the bond length. Sigma bond is formed by head to head overlap. Ember that, pi bond can exist only when the sigma is present. Extent of overlapping decides the strength of bond.
Complete step by step answer:
The covalent bond is formed by the head to head overlap of atomic orbitals. A covalent bond formed is linear or coaxial. The bond formed is in a line internuclear axis of atomic orbitals. This is known as a sigma bond. For example, the s-orbitals undergo the head to head overlap to form a sigma bond as shown.

The covalent bond is formed on the internuclear axis. Let’s consider the example of s orbitals. S-orbitals are spherical and can overlap from any side. The S orbital overlap forms a sigma bond.
1) The Sigma bond is covalent which is formed by the overlap of atomic orbitals.
2) It is possible between two orbitals such as s-s, p-p, or s-p.
3) They have high bond energy.
-A covalent bond that is formed by the lateral or sideway overlapping of pure orbitals is called the pi bond. It is formed by the side to side overlapping of orbitals. There is three types of p orbitals${{P}_{x}},{{P}_{y}}\text{ and }{{\text{P}}_{\text{z}}}$. This is mutually perpendicular to each other. The pi bond is between the p orbitals which are mutually parallel to each other but perpendicular to the internuclear axis. For example, the orbitals ${{p}_{y}}-{{p}_{y}}$overlap to form a pi bond as shown.

1) This is formed by the sideway overlap of atomic orbitals.
2) It exists between two p orbital only.
3) Have less bond energy than sigma bonds.
4) It forms only when the molecule has already formed a sigma bond.
5) It is weak due to the lower extent of overlapping.
Here, the extent of overlapping of the orbital along the internuclear axis is greater than the sideway overlapping of atomic orbitals. We know that, the larger the extent of overlapping the stronger the bond formed. In sigma bonds, the large overlap of the orbital involves the removal of a large amount of energy. While in pi bonds the extent of overlapping is less than sigma bond.
Therefore, sigma bond is stronger than pi bond.
Note: Bond strength depends on the bond length. Sigma bond is formed by head to head overlap. Ember that, pi bond can exist only when the sigma is present. Extent of overlapping decides the strength of bond.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




