
Explain why is \[ - COOC{H_3}\] a meta – directing group while \[ - OCOC{H_3}\] is a o, p – directing group
Answer
582k+ views
Hint: Meta, ortho and para are positions on benzene rings that represent the sites at which functional groups attach themselves. The nature of the functional group determines the position at which it will be placed on the benzene ring.
Complete step by step answer:
Considering ‘Z’ to be the point of reference, we can represent the Meta, ortho and para as follows:
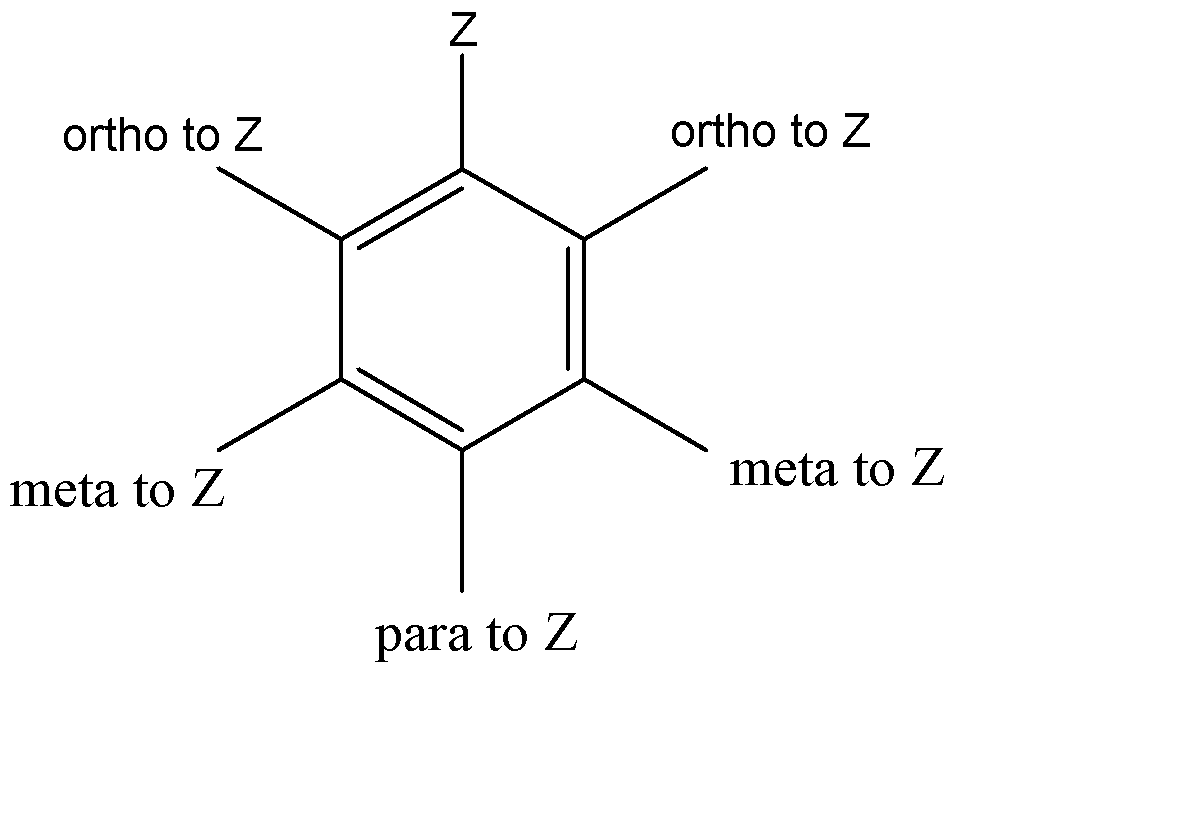
1. \[ - COOC{H_3}\] : \[ - COOC{H_3}\] is an electron withdrawing group. Hence, it would attract electrons from the bonds, which will in turn attract electrons from Z to stabilize. Hence to form stable resonance structures, \[ - COOC{H_3}\] is a meta directing group.
2. \[ - OCOC{H_3}\] : \[ - OCOC{H_3}\] is an electron donating group. Hence it would feed electrons to the bond to increase the number of resonance structures, which would in turn increase the stability of the compound. Hence it will be placed at the ortho and para positions with respect to Z, because then the movement of electrons is not interrupted.
Additional information:
In an electrophilic aromatic substitution reaction, existing substituent groups on the aromatic groups on the aromatic ring influence the overall reaction rate or have a directing effect on the positional isomer of the products that are formed.
Note:
In a situation where the yield of the meta product of a compound is lower than the yield of the ortho or the para compound, then the substituent that is present on the monosubstituted benzene ring is known as ‘ortho, para’ or ‘o, p’ directing group and vice versa.
Complete step by step answer:
Considering ‘Z’ to be the point of reference, we can represent the Meta, ortho and para as follows:

1. \[ - COOC{H_3}\] : \[ - COOC{H_3}\] is an electron withdrawing group. Hence, it would attract electrons from the bonds, which will in turn attract electrons from Z to stabilize. Hence to form stable resonance structures, \[ - COOC{H_3}\] is a meta directing group.
2. \[ - OCOC{H_3}\] : \[ - OCOC{H_3}\] is an electron donating group. Hence it would feed electrons to the bond to increase the number of resonance structures, which would in turn increase the stability of the compound. Hence it will be placed at the ortho and para positions with respect to Z, because then the movement of electrons is not interrupted.
Additional information:
In an electrophilic aromatic substitution reaction, existing substituent groups on the aromatic groups on the aromatic ring influence the overall reaction rate or have a directing effect on the positional isomer of the products that are formed.
Note:
In a situation where the yield of the meta product of a compound is lower than the yield of the ortho or the para compound, then the substituent that is present on the monosubstituted benzene ring is known as ‘ortho, para’ or ‘o, p’ directing group and vice versa.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




