
Explain Williamson’s synthesis with one example.
Answer
571.5k+ views
Hint: To solve this we must know what is Williamson’s synthesis used for. Williamson’s synthesis is used for the synthesis of symmetrical and unsymmetrical ethers from alkyl halides. We should keep in mind that williamsons synthesis involves a reaction which works in the Nucleophilic Substitution mechanism.
Complete step by step solution:
We know that Williamson’s synthesis is used for the synthesis of symmetrical and unsymmetrical ethers.
In Williamson’s synthesis, alkyl halide $\left( {{\text{R}}' - {\text{X}}} \right)$ reacts with sodium alkoxide $\left( {{\text{R}}{{\text{O}}^ - }{\text{N}}{{\text{a}}^ + }} \right)$ to produce ethers $\left( {{\text{R}} - {\text{O}} - {\text{R}}'} \right)$. Sodium halide $\left( {{\text{N}}{{\text{a}}^ + }{{\text{X}}^ - }} \right)$ is the by-product of the reaction.
The general reaction for the production of ethers using Williamson’s synthesis is as follows:
${\text{R}}{{\text{O}}^ - }{\text{N}}{{\text{a}}^ + } + {\text{R}}' - {\text{X}} \to {\text{R}} - {\text{O}} - {\text{R}}' + {\text{N}}{{\text{a}}^ + }{{\text{X}}^ - }$
Williamson’s synthesis is a nucleophilic substitution reaction.
In alkyl halides, a positive charge develops on the carbon atom attached to the halogen atom. This is because the carbon halogen bond is polar in nature due to the high electronegativity of the halogen atom. As a result the carbon atom becomes electrophilic in nature.
The alkoxide ion acts as a nucleophile. The alkoxide ion substitutes the halide ion from the alkyl halide. The reaction is a bimolecular nucleophilic substitution reaction i.e. ${{\text{S}}_{\text{N}}}2$ reaction.
For example: Preparation of ethoxyethane from sodium ethanolate and chloroethane. The reaction is as follows:
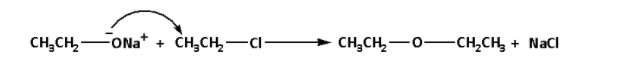
In the reaction, sodium ethanolate reacts with chloroethane and ethoxyethane is produced. The by-product of the reaction is sodium chloride.
Note: The Williamson’s synthesis follows ${{\text{S}}_{\text{N}}}2$ mechanism i.e. bimolecular nucleophilic substitution mechanism. It is a one-step reaction. In the reaction, the bond breaking and bond making occurs simultaneously. No intermediate is formed in the reaction. The nucleophile attacks the alkyl halide from the back side and thus, the product formed has a reversed configuration.
Complete step by step solution:
We know that Williamson’s synthesis is used for the synthesis of symmetrical and unsymmetrical ethers.
In Williamson’s synthesis, alkyl halide $\left( {{\text{R}}' - {\text{X}}} \right)$ reacts with sodium alkoxide $\left( {{\text{R}}{{\text{O}}^ - }{\text{N}}{{\text{a}}^ + }} \right)$ to produce ethers $\left( {{\text{R}} - {\text{O}} - {\text{R}}'} \right)$. Sodium halide $\left( {{\text{N}}{{\text{a}}^ + }{{\text{X}}^ - }} \right)$ is the by-product of the reaction.
The general reaction for the production of ethers using Williamson’s synthesis is as follows:
${\text{R}}{{\text{O}}^ - }{\text{N}}{{\text{a}}^ + } + {\text{R}}' - {\text{X}} \to {\text{R}} - {\text{O}} - {\text{R}}' + {\text{N}}{{\text{a}}^ + }{{\text{X}}^ - }$
Williamson’s synthesis is a nucleophilic substitution reaction.
In alkyl halides, a positive charge develops on the carbon atom attached to the halogen atom. This is because the carbon halogen bond is polar in nature due to the high electronegativity of the halogen atom. As a result the carbon atom becomes electrophilic in nature.
The alkoxide ion acts as a nucleophile. The alkoxide ion substitutes the halide ion from the alkyl halide. The reaction is a bimolecular nucleophilic substitution reaction i.e. ${{\text{S}}_{\text{N}}}2$ reaction.
For example: Preparation of ethoxyethane from sodium ethanolate and chloroethane. The reaction is as follows:

In the reaction, sodium ethanolate reacts with chloroethane and ethoxyethane is produced. The by-product of the reaction is sodium chloride.
Note: The Williamson’s synthesis follows ${{\text{S}}_{\text{N}}}2$ mechanism i.e. bimolecular nucleophilic substitution mechanism. It is a one-step reaction. In the reaction, the bond breaking and bond making occurs simultaneously. No intermediate is formed in the reaction. The nucleophile attacks the alkyl halide from the back side and thus, the product formed has a reversed configuration.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




