
${{[Fe{{(CN)}_{6}}]}^{-3}}$ is a low spin complex but ${{[Fe{{({{H}_{2}}O)}_{6}}]}^{+3}}$ is a high spin complex. Explain
Answer
573k+ views
Hint: A list of ligands based on the strength of their interaction with metal ions is known as the Spectrochemical series. The ligands are often arranged from weaker to stronger ligands
Complete step by step answer:
-The net charge in crystal energy in Crystal field theory which results from the orientation of d-orbitals of a transition metal cation inside a coordinating group of anions are called ligands.
-The field produced by the ligand and the charge on the metal ion determines the type of crystal field splitting that will occur within the complex.
-In simple words, the spectrochemical series is a list of ligands arranged in the order of their field strength. The spectrochemical series ranks the ligands according to the energy difference between the \[{{t}_{2g}}\]and ${{e}_{g}}$ orbitals.
-In 1938 based on the results of the absorption spectra of cobalt complexes, the spectrochemical series was first proposed.
-The spectrochemical series of ligands is as follows-
${{I}^{-}}$ < $B{{r}^{-}}$ < $SC{{N}^{-}}$ < $C{{l}^{-}}$ < ${{S}^{-2}}$ < ${{N}_{3}}^{-}$ < ${{F}^{-}}$ < $ON{{O}^{-}}$ < $O{{H}^{-}}$ < $S{{O}_{4}}^{-2}$ < $N{{O}_{4}}^{-}$ < ${{C}_{2}}{{O}_{4}}^{-2}$ < ${{O}^{-2}}$ < ${{H}_{2}}O\sim NC{{S}^{-}}$ < $EDT{{A}^{-4}}$ < $N{{H}_{3}}\sim Py$ < $en$ < $bipy\sim phen$ < $N{{O}_{2}}^{-}$ < $P{{R}_{3}}$ < $C{{H}_{3}}$ < $C{{N}^{-}}\sim CO$
in which py is pyridine; en is ethylenediamine; bipy is 2,2-bipyridine; phen is 1,10-phenanthroline; SCN
means that the ligand is bound via sulfur and NCS means the ligand is bound via nitrogen.
Ligands from ${{I}^{-}}$ to ${{H}_{2}}O$ are weak field ligands, and ligands from $NC{{S}^{-}}$to CO are strong field ligands.
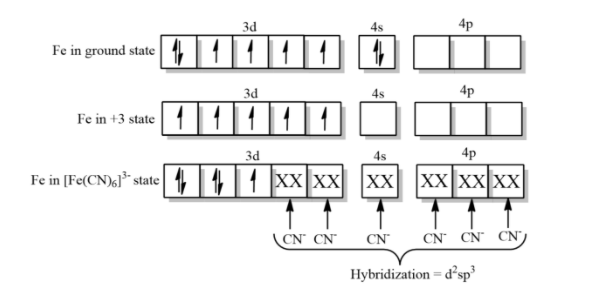
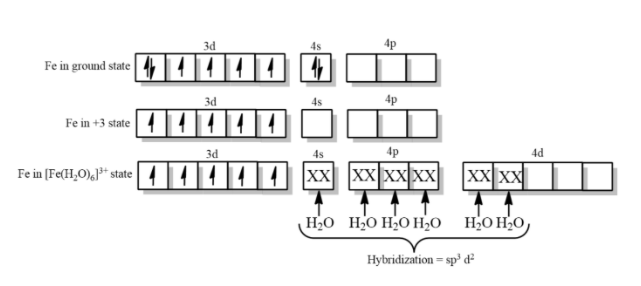
-In complex ${{[Fe{{(CN)}_{6}}]}^{-3}}$, $C{{N}^{-}}$ ligand according to spectrochemical series is a strong field ligand and can pair electrons, but in the complex ${{[Fe{{({{H}_{2}}O)}_{6}}]}^{+3}}$, the ligand ${{H}_{2}}O$is a weak field ligand and thus it cannot pair the electrons.
Therefore, in ${{[Fe{{(CN)}_{6}}]}^{-3}}$, due to the presence of less unpaired electrons with cyanide being a strong field ligand is a high spin complex but ${{[Fe({{H}_{2}}O)]}^{+3}}$is a low spin complex with ${{H}_{2}}O$ being a weak field ligand because of the presence of five unpaired electrons.
Note: Ligands arranged in the left end of the spectrochemical series, that is weak field ligands form outer orbital octahedral complexes of high spin, whereas the ligands lying the right end of the spectrochemical series, that is strong field ligands form inner orbital complexes which means that they are low spin ligands.
Complete step by step answer:
-The net charge in crystal energy in Crystal field theory which results from the orientation of d-orbitals of a transition metal cation inside a coordinating group of anions are called ligands.
-The field produced by the ligand and the charge on the metal ion determines the type of crystal field splitting that will occur within the complex.
-In simple words, the spectrochemical series is a list of ligands arranged in the order of their field strength. The spectrochemical series ranks the ligands according to the energy difference between the \[{{t}_{2g}}\]and ${{e}_{g}}$ orbitals.
-In 1938 based on the results of the absorption spectra of cobalt complexes, the spectrochemical series was first proposed.
-The spectrochemical series of ligands is as follows-
${{I}^{-}}$ < $B{{r}^{-}}$ < $SC{{N}^{-}}$ < $C{{l}^{-}}$ < ${{S}^{-2}}$ < ${{N}_{3}}^{-}$ < ${{F}^{-}}$ < $ON{{O}^{-}}$ < $O{{H}^{-}}$ < $S{{O}_{4}}^{-2}$ < $N{{O}_{4}}^{-}$ < ${{C}_{2}}{{O}_{4}}^{-2}$ < ${{O}^{-2}}$ < ${{H}_{2}}O\sim NC{{S}^{-}}$ < $EDT{{A}^{-4}}$ < $N{{H}_{3}}\sim Py$ < $en$ < $bipy\sim phen$ < $N{{O}_{2}}^{-}$ < $P{{R}_{3}}$ < $C{{H}_{3}}$ < $C{{N}^{-}}\sim CO$
in which py is pyridine; en is ethylenediamine; bipy is 2,2-bipyridine; phen is 1,10-phenanthroline; SCN
means that the ligand is bound via sulfur and NCS means the ligand is bound via nitrogen.
Ligands from ${{I}^{-}}$ to ${{H}_{2}}O$ are weak field ligands, and ligands from $NC{{S}^{-}}$to CO are strong field ligands.
-In complex ${{[Fe{{(CN)}_{6}}]}^{-3}}$, $C{{N}^{-}}$ ligand according to spectrochemical series is a strong field ligand and can pair electrons, but in the complex ${{[Fe{{({{H}_{2}}O)}_{6}}]}^{+3}}$, the ligand ${{H}_{2}}O$is a weak field ligand and thus it cannot pair the electrons.
Therefore, in ${{[Fe{{(CN)}_{6}}]}^{-3}}$, due to the presence of less unpaired electrons with cyanide being a strong field ligand is a high spin complex but ${{[Fe({{H}_{2}}O)]}^{+3}}$is a low spin complex with ${{H}_{2}}O$ being a weak field ligand because of the presence of five unpaired electrons.


Note: Ligands arranged in the left end of the spectrochemical series, that is weak field ligands form outer orbital octahedral complexes of high spin, whereas the ligands lying the right end of the spectrochemical series, that is strong field ligands form inner orbital complexes which means that they are low spin ligands.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




