
${{[Fe{{F}_{6}}]}^{3-}}$ has Fe atom _________hybridized with unpaired_______electrons.
(A) ${{d}^{2}}s{{p}^{3}}$ , 4
(B) ${{d}^{2}}s{{p}^{3}}$ , 5
(C) $s{{p}^{3}}{{d}^{2}}$, 5
(D) $s{{p}^{3}}{{d}^{2}}$, 3
Answer
585k+ views
Hint: The hybridization of the inorganic complexes is going to depend on the type of ligand. If the ligand is a strong ligand then the inner orbital complex is going to form and if the ligand is weak then the outer orbital complex is going to form.
Complete step by step solution:
-In ${{[Fe{{F}_{6}}]}^{3-}}$ one iron atom and six fluorine atoms are there.
-We know that fluorine is a weak ligand and forms an outer orbital complex with iron.
-The oxidation state of iron in ${{[Fe{{F}_{6}}]}^{3-}}$ is as follows.
x + 6(-1) = -3
x – 6 = -3
x = 3
Here x = oxidation state of iron
-The electronic configuration of iron is as follows.
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{6}}4{{p}^{0}}4{{d}^{0}}\]
-The electronic configuration of iron in ${{[Fe{{F}_{6}}]}^{3-}}$ is as follows.
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{0}}3{{d}^{5}}4{{p}^{0}}4{{d}^{0}}\]
-Six fluorine atoms donate electrons to iron.
-The hybridization of Fe atom in ${{[Fe{{F}_{6}}]}^{3-}}$ is as follows.
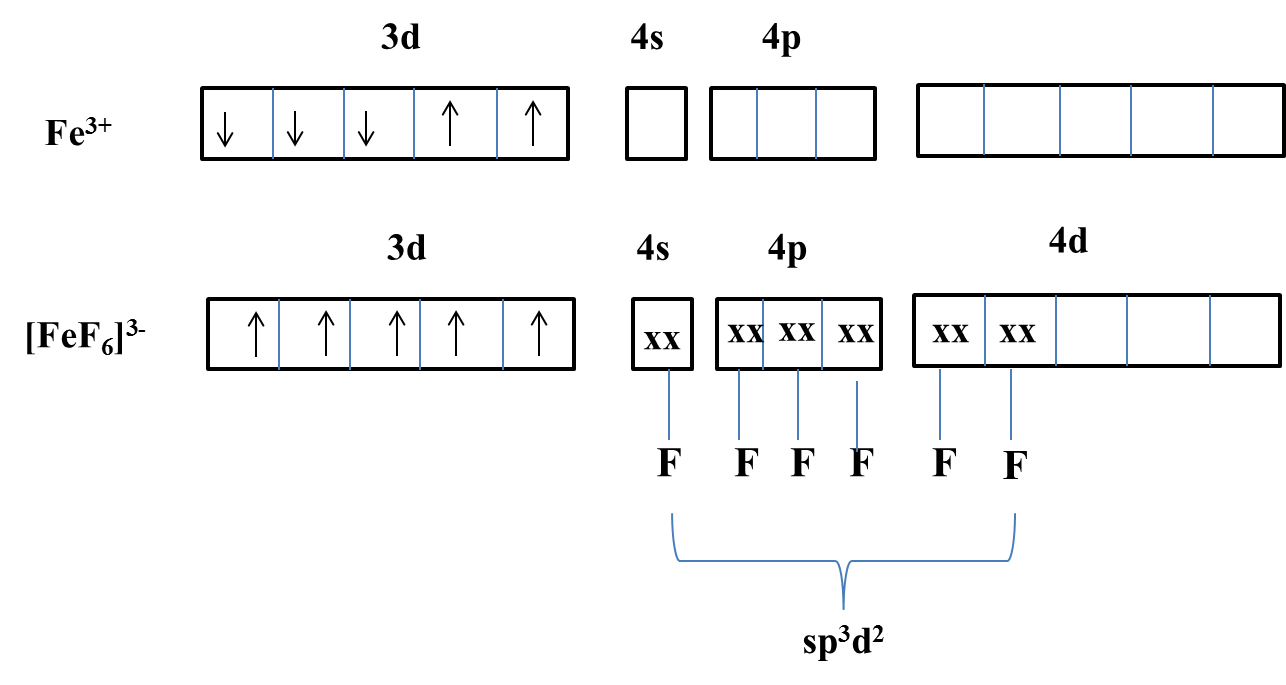
-From the above picture we can say that iron undergoes $s{{p}^{3}}{{d}^{2}}$ hybridization.
-From the above picture, we can also say that iron in ${{[Fe{{F}_{6}}]}^{3-}}$ have 5 unpaired electrons.
-Therefore the hybridization of iron in ${{[Fe{{F}_{6}}]}^{3-}}$ is $s{{p}^{3}}{{d}^{2}}$ with 5 unpaired electrons.
So, the correct option is (C).
Note: An inorganic metal complex in which the central metal atom utilizes outer d orbitals for hybridization then the complex is called outer orbital complex. An inorganic metal complex in which the central metal atom utilizes inner d orbitals for hybridization then the complex is called inner orbital complex.
Complete step by step solution:
-In ${{[Fe{{F}_{6}}]}^{3-}}$ one iron atom and six fluorine atoms are there.
-We know that fluorine is a weak ligand and forms an outer orbital complex with iron.
-The oxidation state of iron in ${{[Fe{{F}_{6}}]}^{3-}}$ is as follows.
x + 6(-1) = -3
x – 6 = -3
x = 3
Here x = oxidation state of iron
-The electronic configuration of iron is as follows.
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{6}}4{{p}^{0}}4{{d}^{0}}\]
-The electronic configuration of iron in ${{[Fe{{F}_{6}}]}^{3-}}$ is as follows.
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{0}}3{{d}^{5}}4{{p}^{0}}4{{d}^{0}}\]
-Six fluorine atoms donate electrons to iron.
-The hybridization of Fe atom in ${{[Fe{{F}_{6}}]}^{3-}}$ is as follows.

-From the above picture we can say that iron undergoes $s{{p}^{3}}{{d}^{2}}$ hybridization.
-From the above picture, we can also say that iron in ${{[Fe{{F}_{6}}]}^{3-}}$ have 5 unpaired electrons.
-Therefore the hybridization of iron in ${{[Fe{{F}_{6}}]}^{3-}}$ is $s{{p}^{3}}{{d}^{2}}$ with 5 unpaired electrons.
So, the correct option is (C).
Note: An inorganic metal complex in which the central metal atom utilizes outer d orbitals for hybridization then the complex is called outer orbital complex. An inorganic metal complex in which the central metal atom utilizes inner d orbitals for hybridization then the complex is called inner orbital complex.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




