
Ferric sulphate is represented by which formula?
A. $FeS{{O}_{4}}$
B. $FeS{{O}_{3}}$
C. \[Fe{{\left( S{{O}_{4}} \right)}_{2}}\]
D. \[F{{e}_{2}}{{\left( S{{O}_{4}} \right)}_{3}}\]
Answer
585.3k+ views
Hint: In order to mention the formula of ferric sulphate we are required to know the structure. Recall that the word ‘ferric’ indicates that the oxidation state of the $Fe$ atom is +3.
Complete step by step answer:
First, we should look at the oxidation states of ions involved in the making of this molecule. The word ferric indicates that the oxidation state of the $Fe$ ion is +3 and the word sulphate indicates that the formula of the ion present is $S{{O}_{4}}$ we know that this ion has an oxidation state of -2 so we can rule out ‘option B’ since it has the $S{{O}_{3}}$ ion. We know that the overall charge on the molecule is 0, so we can balance the charges to find the number of each ion involved in the molecule.
We see by trial and error method by considering all the options given that only in option ‘D. \[F{{e}_{2}}{{\left( S{{O}_{4}} \right)}_{3}}\]’ the overall charge comes out to be 0 (It is +1 in option A and -1 in option C).
The structure of Ferric sulphate is given below:
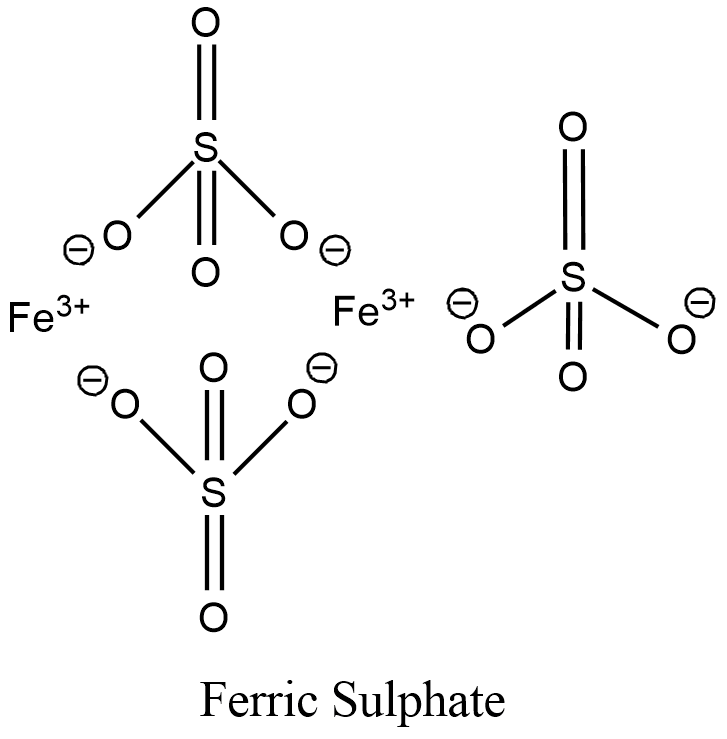
Hence, the answer is. \[F{{e}_{2}}{{\left( S{{O}_{4}} \right)}_{3}}\]’.
So, the correct answer is “Option B”.
Note: Remember that when the ion $S{{O}_{3}}^{2-}$ is involved, the word sulphite will be used in the naming of the molecule. The word that is indicative of the $F{{e}^{2+}}$ ion is ferrous. Keep this in mind while answering the questions.
Complete step by step answer:
First, we should look at the oxidation states of ions involved in the making of this molecule. The word ferric indicates that the oxidation state of the $Fe$ ion is +3 and the word sulphate indicates that the formula of the ion present is $S{{O}_{4}}$ we know that this ion has an oxidation state of -2 so we can rule out ‘option B’ since it has the $S{{O}_{3}}$ ion. We know that the overall charge on the molecule is 0, so we can balance the charges to find the number of each ion involved in the molecule.
We see by trial and error method by considering all the options given that only in option ‘D. \[F{{e}_{2}}{{\left( S{{O}_{4}} \right)}_{3}}\]’ the overall charge comes out to be 0 (It is +1 in option A and -1 in option C).
The structure of Ferric sulphate is given below:

Hence, the answer is. \[F{{e}_{2}}{{\left( S{{O}_{4}} \right)}_{3}}\]’.
So, the correct answer is “Option B”.
Note: Remember that when the ion $S{{O}_{3}}^{2-}$ is involved, the word sulphite will be used in the naming of the molecule. The word that is indicative of the $F{{e}^{2+}}$ ion is ferrous. Keep this in mind while answering the questions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




