
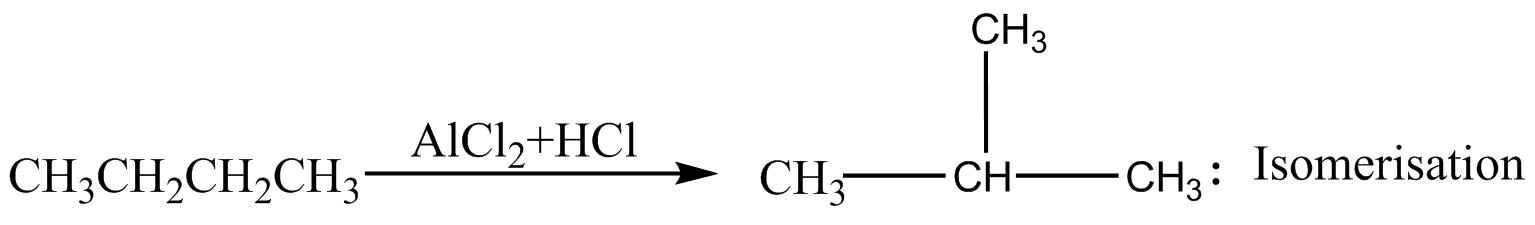
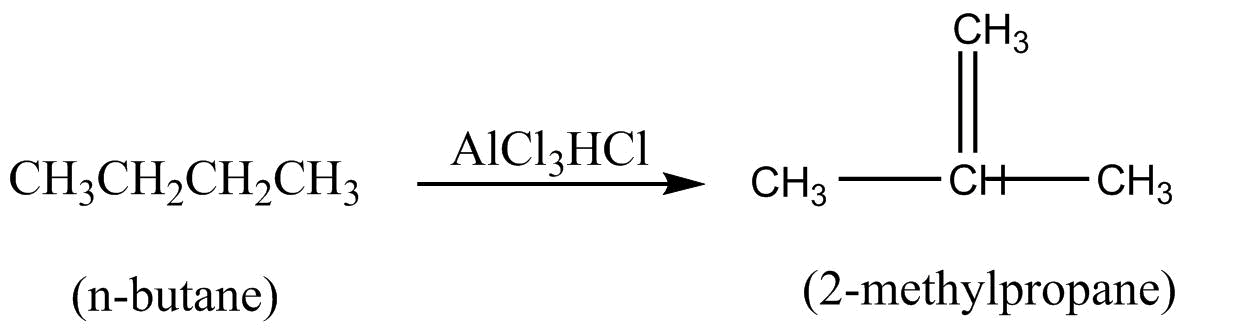
Few reactions of alkanes are given below. The name of the reaction is not correctly with the reaction is :
A.
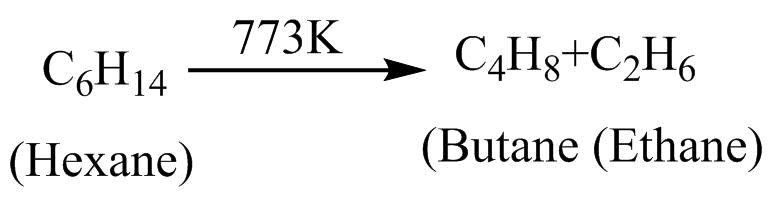
B. \[{C_6}{H_{14}}\xrightarrow{{773K}}{C_4}{H_8} + {C_2}{H_6}:{\text{Pyrolysis}}\]
C. \[C{H_4} + {20_2}\xrightarrow{\Delta }C{O_2} + 2{H_2}O:{\text{ Controlled oxidation}}\]
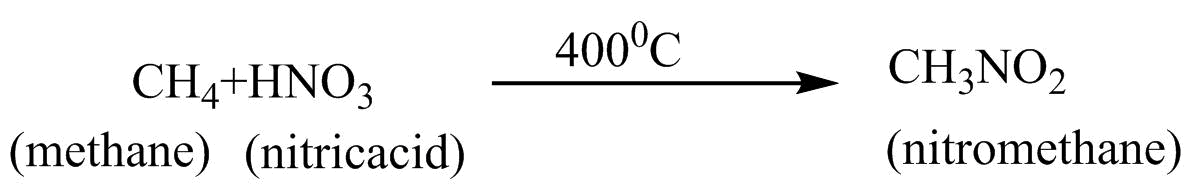
D. $CH_{4}$ + $HNO_{3}$ $\xrightarrow{{400^{o}C}}$ $CH_{3}NO_{2}$ $Nitration$

Answer
576.3k+ views
Hint: Organic chemistry is a branch of chemistry that deals with study of structures, reactions, properties , preparations, composition of various carbon containing compounds.
Isomerization, pyrolysis, controlled oxidation and nitration are a few reactions to names that are studied in organic chemistry.
Complete answer:
Isomerization :- It is a phenomenon or reaction in which two or more compounds have the same mole aver formula, known as isomers .
In option A, $n - $Butane is getting converted into $2 - $methyl prone. Both the compounds have the same molecular formula but different structures. Therefore, they are undergoing isomerization.
Pyrolysis :- The breaking down of hydrocarbon chains into simpler hydrocarbons by applying heat is known as pyrolysis In option B, Hexone is broken down into Butane and ethane. Therefore it is showing pyrolysis.
Controlled oxidation :-
Controlled oxidation is a process in which during storage, the chemicals are stored with roble gas.
The products of controlled oxidation are formaldehyde and water.
But, in option (C), the products formed are carbon dioxide and water :-
$C{H_4} + 2{O_2}\xrightarrow{\Delta }C{O_2} + 2{H_2}O$
This reaction is representing complete oxidation.
Actual reaction of controlled oxidation would be
$C{H_4} + {O_2} \to HCHO + {H_2}O\;({\text{fomaldehyole}})$
(D) Nitration :- The addition of nitro groups in the presence of nitric acid on a compound is known as nitration.
In option D, nitro $\left( { - N{O_2}} \right)$ group is being added on methane in presence of $HN{O_3}$. Therefore, it is a nitration reaction.
Hence, the correct option is (C). \[C{H_4} + {20_2}\xrightarrow{\Delta }C{O_2} + 2{H_2}O:{\text{ Controlled oxidation}}\]
Note: All the reaction have fixed specific temperature of a reaction always remain the same, only the number of elements in the compounds may differ
Isomerization, pyrolysis, controlled oxidation and nitration are a few reactions to names that are studied in organic chemistry.
Complete answer:
Isomerization :- It is a phenomenon or reaction in which two or more compounds have the same mole aver formula, known as isomers .
In option A, $n - $Butane is getting converted into $2 - $methyl prone. Both the compounds have the same molecular formula but different structures. Therefore, they are undergoing isomerization.

Pyrolysis :- The breaking down of hydrocarbon chains into simpler hydrocarbons by applying heat is known as pyrolysis In option B, Hexone is broken down into Butane and ethane. Therefore it is showing pyrolysis.

Controlled oxidation :-
Controlled oxidation is a process in which during storage, the chemicals are stored with roble gas.
The products of controlled oxidation are formaldehyde and water.
But, in option (C), the products formed are carbon dioxide and water :-
$C{H_4} + 2{O_2}\xrightarrow{\Delta }C{O_2} + 2{H_2}O$
This reaction is representing complete oxidation.
Actual reaction of controlled oxidation would be
$C{H_4} + {O_2} \to HCHO + {H_2}O\;({\text{fomaldehyole}})$
(D) Nitration :- The addition of nitro groups in the presence of nitric acid on a compound is known as nitration.
In option D, nitro $\left( { - N{O_2}} \right)$ group is being added on methane in presence of $HN{O_3}$. Therefore, it is a nitration reaction.

Hence, the correct option is (C). \[C{H_4} + {20_2}\xrightarrow{\Delta }C{O_2} + 2{H_2}O:{\text{ Controlled oxidation}}\]
Note: All the reaction have fixed specific temperature of a reaction always remain the same, only the number of elements in the compounds may differ
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




