
Few statements are given regarding nodes in the orbitals. Mark the statement which is not correct.
(A) In case of P, orbital, xy plane is a nodal plane
(B) ns orbital has $(n+1)$ nodes.
(C) The number of angular nodes is given by l.
(D) The total number of nodes is given by $(n-1)$ i.e. angular nodes and in $(n-l-1)$ radial nodes.
Answer
564.9k+ views
Hint To solve this question first we have to understand the term orbitals. Orbitals are 3 dimensional space around the nucleus where the probability of finding an electron is maximum.
Complete solution:
The p orbital is also an atomic orbital and the shape of p orbital is dumbbell shape. The energy levels of p orbitals are higher as compared to that of p orbitals. Angular nodes are present in the p orbitals. The maximum number of electrons which can be present in the p orbital is 6. P orbitals have 3 sub orbitals. The value of angular momentum quantum number for p orbitals is 1. There are lobes present in the p orbital.
The shape of p orbitals is mentioned below:
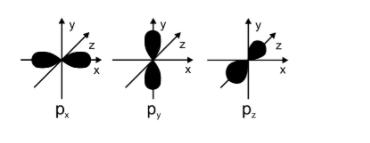
In case of the ${{p}_{z}}$ orbitals, the lobes are present along the z-axis and thus the nodal plane in this case is $x-y$ . for ns orbitals the total number of nodes will be equal to $n-1$
The number of angular nodes = 1 and the number of radial nodes = $n-1-1$
Hence, the total number of nodes will be equal to the sum of angular nodes and radial nodes i.e. $n-1-1+1=n-1$.
Hence, the correct answer is option (B).
Note: Do not get confused between the term orbit and orbitals. Both orbit and orbitals have different meanings as we know that orbitals are 3 dimensional space around the nucleus where the probability of finding an electron is maximum and orbits are the well-defined circular paths in which electrons revolve.
Complete solution:
The p orbital is also an atomic orbital and the shape of p orbital is dumbbell shape. The energy levels of p orbitals are higher as compared to that of p orbitals. Angular nodes are present in the p orbitals. The maximum number of electrons which can be present in the p orbital is 6. P orbitals have 3 sub orbitals. The value of angular momentum quantum number for p orbitals is 1. There are lobes present in the p orbital.
The shape of p orbitals is mentioned below:

In case of the ${{p}_{z}}$ orbitals, the lobes are present along the z-axis and thus the nodal plane in this case is $x-y$ . for ns orbitals the total number of nodes will be equal to $n-1$
The number of angular nodes = 1 and the number of radial nodes = $n-1-1$
Hence, the total number of nodes will be equal to the sum of angular nodes and radial nodes i.e. $n-1-1+1=n-1$.
Hence, the correct answer is option (B).
Note: Do not get confused between the term orbit and orbitals. Both orbit and orbitals have different meanings as we know that orbitals are 3 dimensional space around the nucleus where the probability of finding an electron is maximum and orbits are the well-defined circular paths in which electrons revolve.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




