
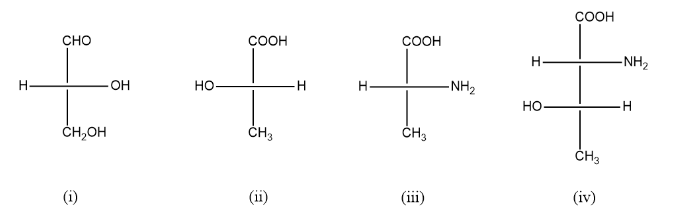
Find R and S configuration of the following compounds.

Answer
513k+ views
Hint : It is easy to figure out which of the compounds is R configuration or S configuration. Just assign $1,2,3,4$to the groups and if it rotates clockwise, it is R; if it rotates anti-clockwise, then it is S configuration. Just remember that the position of hydrogen is always away from the viewer.
Complete Step By Step Answer:
The R and S configuration is a nomenclature used for naming enantiomers of a chiral compound. Enantiomers are the pair of compounds that are mirror images of each other. The R and S system of naming is referred to as the CIP or Cahn Ingold Prelog system. It was proposed by Robert Cahn, Chris Ingold, and Vladimir Prelog in 1966. This system is also known as the absolute configuration of chiral compounds.
There are certain rules given by Cahn Ingold and Prelog to assign R and S names to the compounds. The rules are as follows:
Give priorities to every atom connected to the chiral center based on their atomic number. Higher priority will be given to those having higher atomic numbers.
Align the chiral center in such a way that the least prioritized atom is pointing away from the viewer.
Finally track down the path of the atoms according to the priority. This means to draw an arrow starting from priority $1$then move to $2$and finally$3$. For this part, you can ignore the ${4^{th}}$prioritized atom. Now if the arrow is going clockwise then the absolute configuration is R and the chiral center will be assigned R. And if the arrow is anti-clockwise then the absolute configuration is S and the chiral center will be assigned S.
One important point you have to remember is that if the lowest priority group is on the vertical line then assign the compounds as said clockwise-R counterclockwise-S but if the lowest priority group is on the horizontal line then the compounds will be assigned opposite configuration.
Now we will apply the above rules to assign R and S configuration to the following compounds
For the first compound:
The atoms for the first compound will be given priority in the following manner:
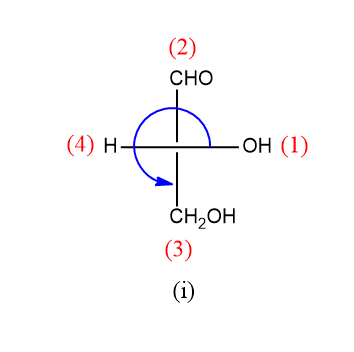
As we can see above, the rotation is anticlockwise but the lowest priority group is on the horizontal line hence its configuration is R.
For the second compound:
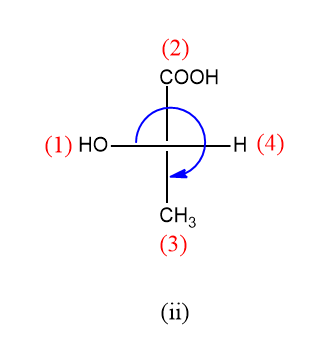
The rotation here is clockwise but the lowest priority group is on the horizontal line so the configuration will be S.
For the third compound:
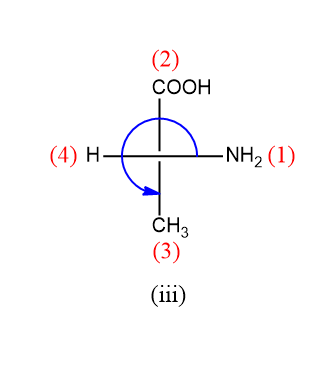
Rotation is anticlockwise but the lowest priority group is on the horizontal line so the configuration will be R.
Now for the last compound, as we can see it has two chiral centers hence both will be given configuration separately as below:
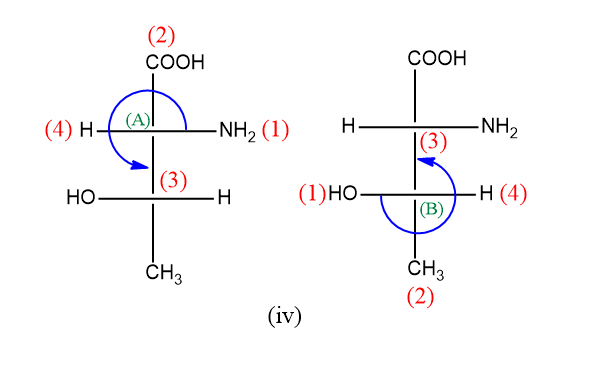
Here for both the chiral center (A) and (B) the rotation is anticlockwise but the lowest priority group is on the horizontal line so the configuration will be R.
Note :
It is important to note that if the lowest priority is on the dash line meaning if it goes backwards (behind the plane) then in clockwise rotation and configuration is R and for anticlockwise the configuration is S and if the lowest priority group is on the wedge line meaning if it comes forward (in front of the plane) then in clockwise rotation the configuration is S and for anticlockwise the configuration is R.
Complete Step By Step Answer:
The R and S configuration is a nomenclature used for naming enantiomers of a chiral compound. Enantiomers are the pair of compounds that are mirror images of each other. The R and S system of naming is referred to as the CIP or Cahn Ingold Prelog system. It was proposed by Robert Cahn, Chris Ingold, and Vladimir Prelog in 1966. This system is also known as the absolute configuration of chiral compounds.
There are certain rules given by Cahn Ingold and Prelog to assign R and S names to the compounds. The rules are as follows:
Give priorities to every atom connected to the chiral center based on their atomic number. Higher priority will be given to those having higher atomic numbers.
Align the chiral center in such a way that the least prioritized atom is pointing away from the viewer.
Finally track down the path of the atoms according to the priority. This means to draw an arrow starting from priority $1$then move to $2$and finally$3$. For this part, you can ignore the ${4^{th}}$prioritized atom. Now if the arrow is going clockwise then the absolute configuration is R and the chiral center will be assigned R. And if the arrow is anti-clockwise then the absolute configuration is S and the chiral center will be assigned S.
One important point you have to remember is that if the lowest priority group is on the vertical line then assign the compounds as said clockwise-R counterclockwise-S but if the lowest priority group is on the horizontal line then the compounds will be assigned opposite configuration.
Now we will apply the above rules to assign R and S configuration to the following compounds
For the first compound:
The atoms for the first compound will be given priority in the following manner:

As we can see above, the rotation is anticlockwise but the lowest priority group is on the horizontal line hence its configuration is R.
For the second compound:

The rotation here is clockwise but the lowest priority group is on the horizontal line so the configuration will be S.
For the third compound:

Rotation is anticlockwise but the lowest priority group is on the horizontal line so the configuration will be R.
Now for the last compound, as we can see it has two chiral centers hence both will be given configuration separately as below:

Here for both the chiral center (A) and (B) the rotation is anticlockwise but the lowest priority group is on the horizontal line so the configuration will be R.
Note :
It is important to note that if the lowest priority is on the dash line meaning if it goes backwards (behind the plane) then in clockwise rotation and configuration is R and for anticlockwise the configuration is S and if the lowest priority group is on the wedge line meaning if it comes forward (in front of the plane) then in clockwise rotation the configuration is S and for anticlockwise the configuration is R.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




